N-containing active center metal organic catalyst for synthesizing cyclic carbonate as well as preparation method and application of N-containing active center metal organic catalyst
A cyclic carbonate and active center technology, applied in the field of N-containing active center organometallic catalysts and its preparation, can solve the problems of lengthy synthetic routes, recovery of complexes, low number of reuses, and not suitable for use in large-scale production , to achieve the effect of safe and simple operation method, good catalytic effect and good industrial application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
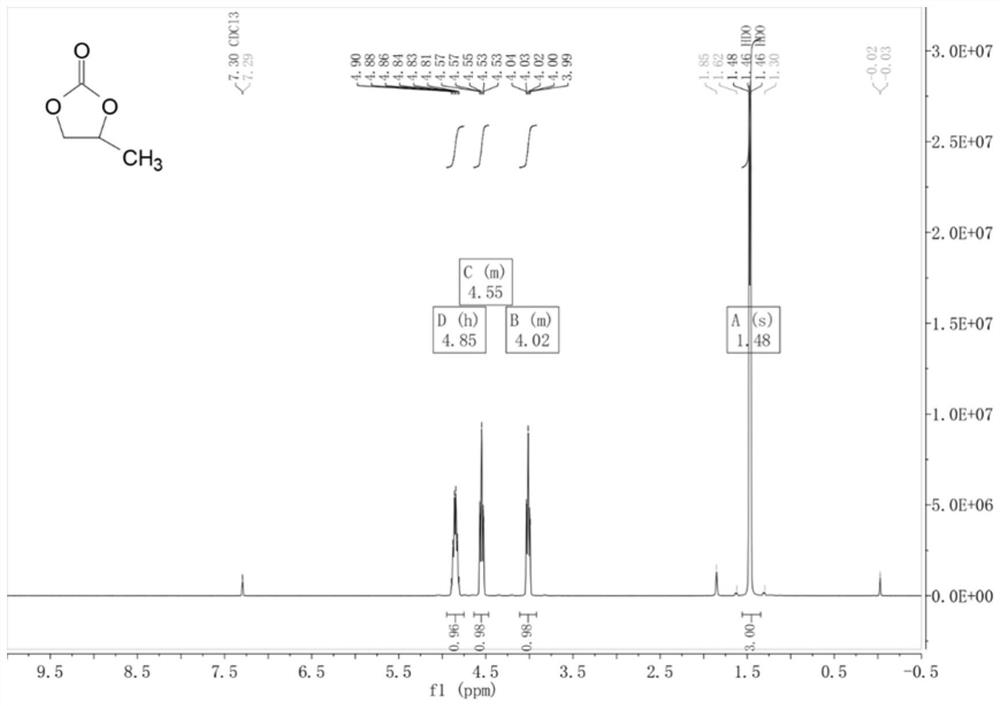
Examples
Embodiment 1
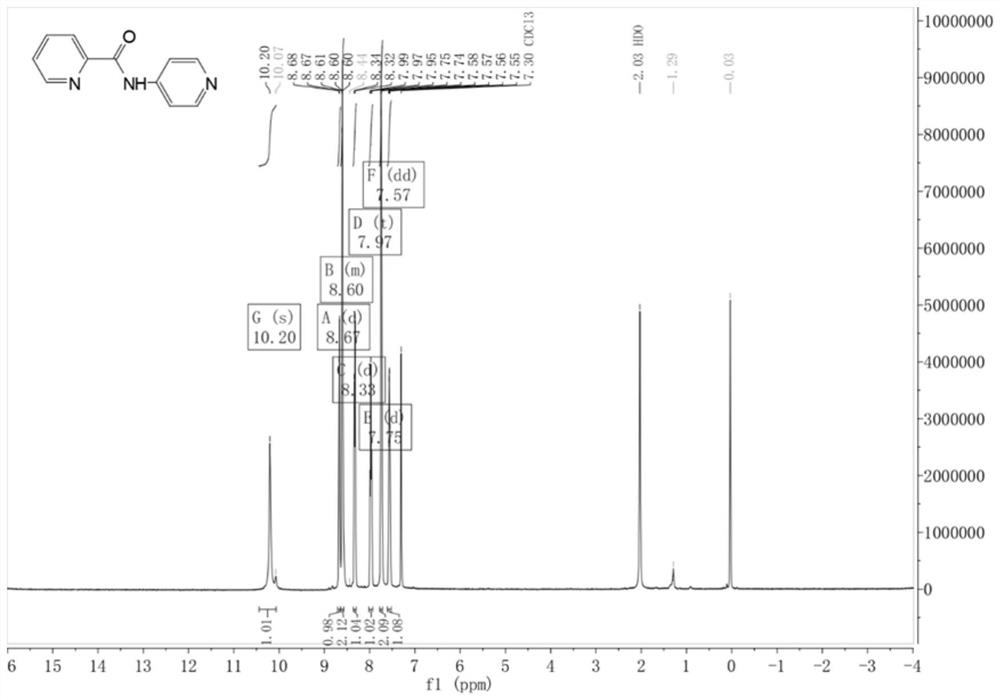
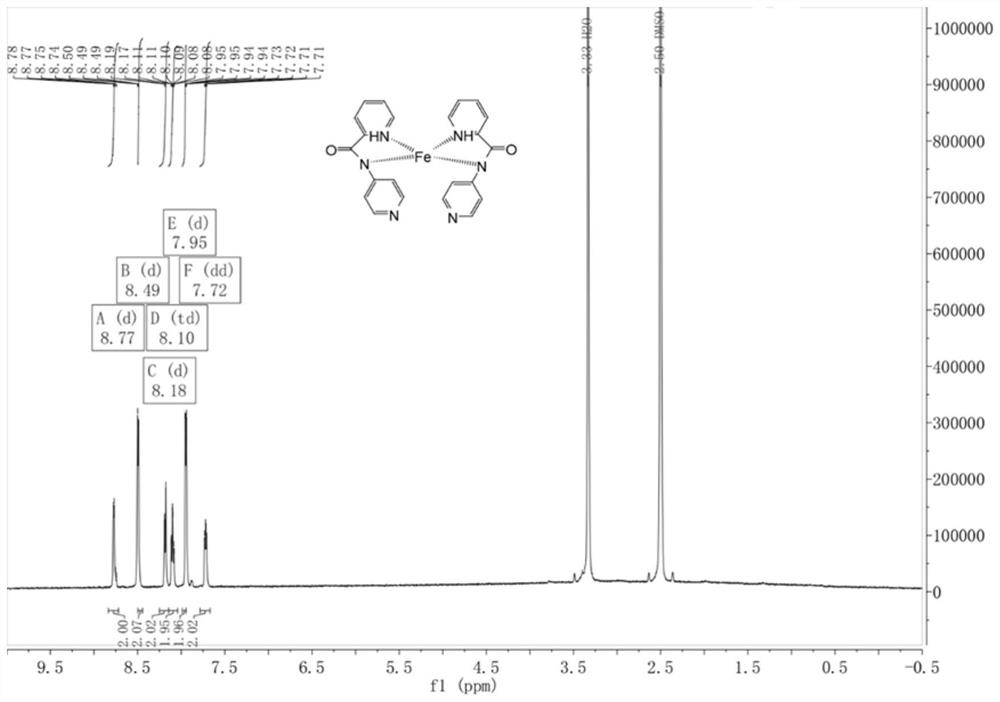
[0061] Weigh 3.16 g (20 mmol) of pyridine-2-carboxylate hydrochloride into a 100 mL round-bottomed flask containing 50 mL of dichloromethane, add 15 mL of thionyl chloride and drop 3 drops of DMF, and reflux at 65°C for 3 hours, the reaction ends Then spin to dry in vacuo to obtain the crude intermediate pyridine-2-acyl chloride, add 50 mL of DCM to the crude pyridine-2-acyl chloride to dissolve to obtain a solution of pyridine-2-acyl chloride in DCM for later use; take 1.88 g of 2-aminopyridine and add it to 100 mL of circular In the bottom flask, 50 mL of solvent DCM and 10 mL of TEA were slowly added dropwise to the DCM solution of pyridine-2-acyl chloride at 0 °C, and stirred at room temperature for 2 h. After the crude ligand was prepared, extraction was performed, and water (50 mL) was added to it. After the layers were separated, the aqueous phase was washed with DCM (30 mL), the combined organic phases were washed with saturated brine (40 mL), and Na 2 SO 4 After dryi...
Embodiment 2
[0065] Weigh 3.16 g (20 mmol) of pyridine-2-carboxylate hydrochloride into a 100 mL round-bottomed flask containing 50 mL of dichloromethane, add 15 mL of thionyl chloride and drop 3 drops of DMF, and reflux at 65°C for 3 hours, the reaction ends Then spin to dry in vacuo to obtain the crude intermediate pyridine-2-acyl chloride, add 50 mL of DCM to the crude pyridine-2-acyl chloride to dissolve the solution to obtain a solution of pyridine-2-acyl chloride in DCM for use; take 1.88 g of 3-aminopyridine and add it to 100 mL of circular In the bottom flask, 50 mL of solvent DCM and 10 mL of TEA were slowly added dropwise to the DCM solution of pyridine-2-acyl chloride at 0 °C, and stirred at room temperature for 2 h. After the crude ligand was prepared, extraction was performed, and water (50 mL) was added to it. After the layers were separated, the aqueous phase was washed with DCM (30 mL), the combined organic phases were washed with saturated brine (40 mL), and Na 2 SO 4 Aft...
Embodiment 3
[0069] Weigh 3.16 g (20 mmol) of pyridine-2-carboxylate hydrochloride into a 100 mL round-bottomed flask containing 50 mL of dichloromethane, add 15 mL of thionyl chloride and drop 3 drops of DMF, and reflux at 65°C for 3 hours, the reaction ends Then spin-dried in vacuo to obtain the crude intermediate pyridine-2-acyl chloride, add 50 mL of DCM to the crude pyridine-2-acyl chloride to obtain a solution of pyridine-2-acyl chloride in DCM for use; take 1.88 g of 4-aminopyridine, add 100 mL In a round-bottomed flask, 50 mL of solvent DCM and 10 mL of TEA were slowly added dropwise to the DCM solution of pyridine-2-acyl chloride at 0 °C, and stirred at room temperature for 2 h. After the crude ligand was prepared, extraction was performed, and water (50 mL) was added to it. After the layers were separated, the aqueous phase was washed with DCM (30 mL), the combined organic phases were washed with saturated brine (40 mL), and Na 2 SO 4 After drying, it was filtered, concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com