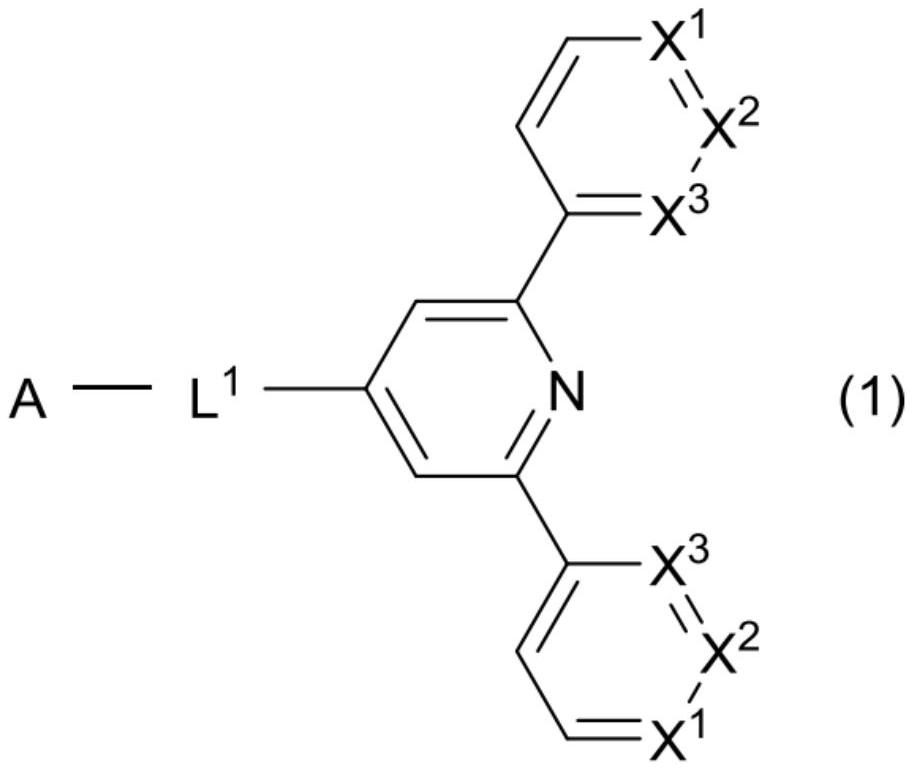
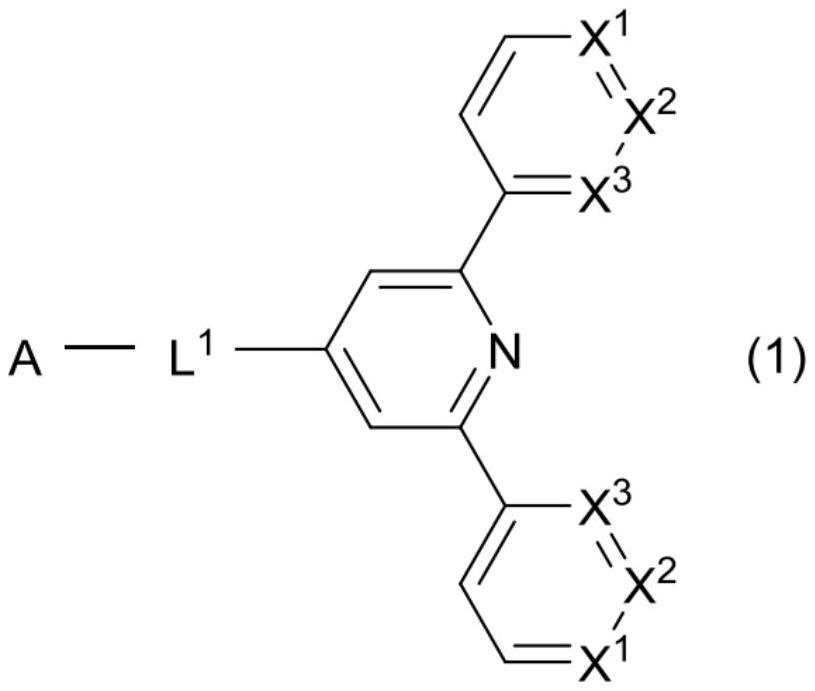
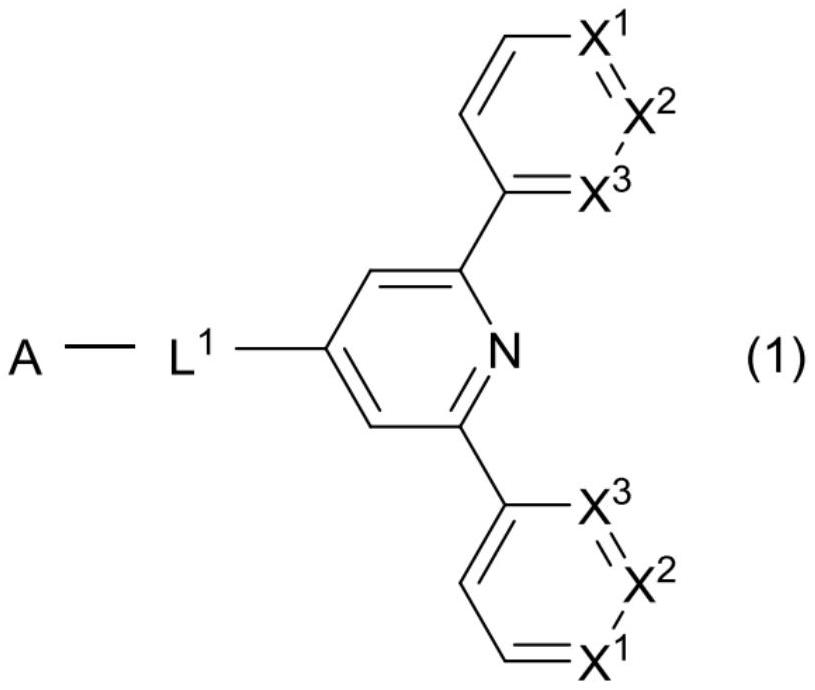
Compound, organic thin-film light-emitting element, display device, and lighting device
A technology of light-emitting element and organic thin film, which is applied in the field of lighting devices to achieve the effect of excellent luminous efficiency and durable life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0140] Hereinafter, the present invention will be described with reference to Examples, but the present invention is not limited to these Examples.
Synthetic example 1
[0141] Synthesis Example 1: Synthesis of Compound 1
[0142] [Chemical 18]
[0143]
[0144] 2.7 g of 4'-(4-bromophenyl)-2,2':6'2"-terpyridine, 1.6 g of 4-(1-pyrene)phenylboronic acid, dichlorobis(triphenylphosphine palladium) The mixed solution of dichloride 50mg, 1.5M sodium carbonate aqueous solution 6ml and dimethoxyethane 70ml was heated and stirred for 5 hours under nitrogen flow and reflux. After cooling to room temperature, water was added and filtered, and methanol was used to clean, The obtained solid was purified by silica gel column chromatography, the solvent was evaporated and the solvent was removed, and the obtained solid was vacuum dried to obtain 2.3 g of Compound 1.
[0145] For the resulting compound 1, use an oil diffusion pump at 1 x 10 -3 It was used in an Example after carrying out sublimation refinement|purification at about 340 degreeC under the pressure of Pa. The high performance liquid chromatography (HPLC) purity (area % at measurement wavel...
Synthetic example 2
[0149] Synthesis Example 2: Synthesis of Compound 2
[0150] [Chemical 19]
[0151]
[0152] 4'-(4-bromophenyl)-2,2':6'2"-terpyridine 4.0 g, 3-chlorophenylboronic acid 1.7 g, dichlorobis(triphenylphosphine palladium) dichloride 72 mg The mixed solution of 10ml of 1.5M sodium carbonate aqueous solution and 70ml of dimethoxyethane was heated and stirred for 3 hours under nitrogen flow and reflux. After cooling to room temperature, water was added and filtered, washed with methanol, and vacuum-dried , 4.0 g of Intermediate A were obtained.
[0153] Next, a mixed solution of 2.9 g of intermediate A, 1.8 g of 1-pyrene boronic acid, 50 mg of dichlorobis(triphenylphosphine palladium) dichloride, 6 ml of a 1.5M aqueous solution of tripotassium phosphate and 40 ml of 1,4-dioxane was prepared. The mixture was heated and stirred under reflux for 7 hours under nitrogen flow. After cooling to room temperature, water was added, followed by filtration, washing with methanol, and vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com