Oral insulin liposome hydrogel and application thereof
An insulin and liposome technology, applied in the field of insulin pharmacy, can solve the problems of easy degradation, poor absorption, and low bioavailability of oral insulin, and achieve the effects of high stability, improved protection, and prolonged residence time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of modified sodium alginate
[0049] Dissolve 1 g of sodium alginate (Alg) in deionized water at a concentration of 20 mg / mL, add NHS and EDC to activate the carboxyl group for 10 min according to the ratio in Table 1, then add cysteine hydrochloride, and stir for 5 After the reaction, the insulin complex was put into a dialysis bag, dialyzed twice with 5 mM HCl solution, 5 mM HCl solution containing 0.9 wt% NaCl, and 1 mM HCl at 4 °C in the dark, and then the pH was adjusted to 4.5 and then freeze-dried. , to obtain modified sodium alginate (cysteine-sodium alginate modification) (Cys-Alg for short).
[0050] Table 1
[0051]
Embodiment 1
[0053] The modified sodium alginate (Cys-Alg) obtained in Example 1 was subjected to Fourier transform infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy, elemental analysis and sulfhydryl content determination:
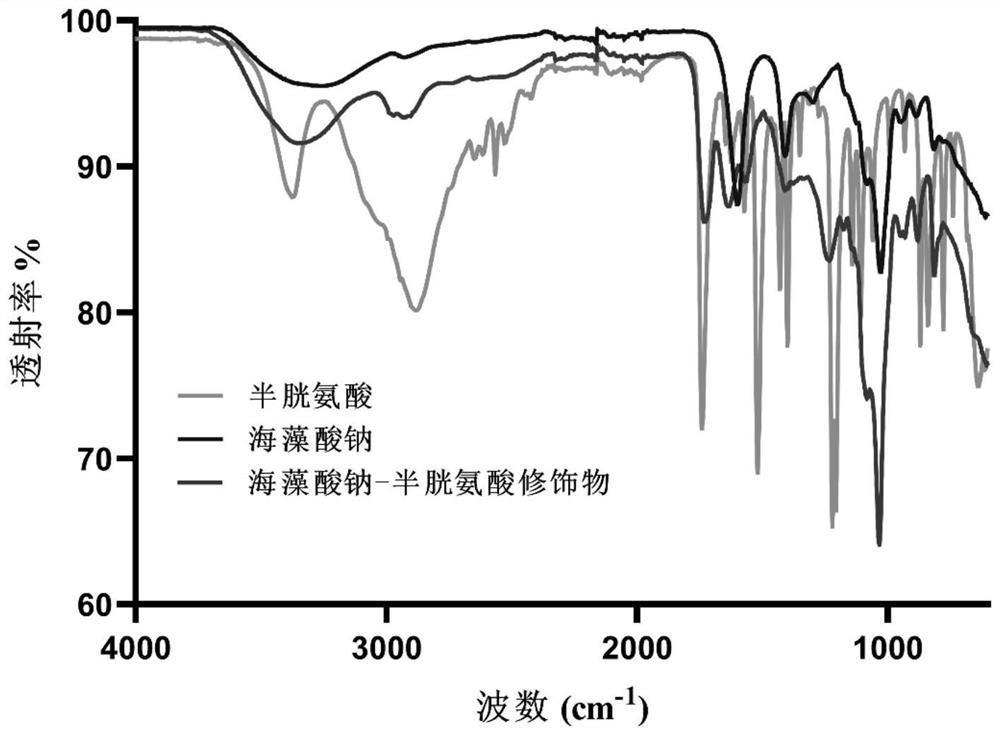
[0054] Fourier transform infrared spectroscopy: Sodium alginate (Alg), cysteine (Cys) and modified sodium alginate (Cys-Alg) were lyophilized and analyzed by ATR-FTIR (Waltham, MA, USA). The frequency range of ATR-FTIR is 4000cm -1 -600cm -1 , the resolution is 4cm -1 , a total of 16 scans, the results are as follows figure 1 shown.
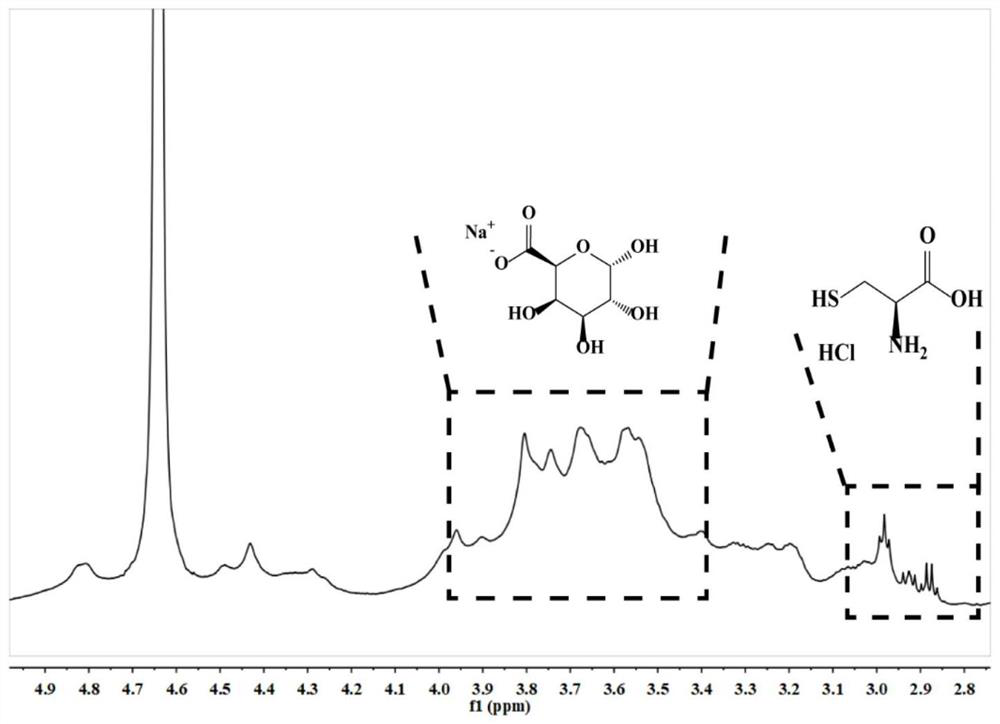
[0055] Hydrogen nuclear magnetic resonance spectrum: Dissolve Alg, Cys-Alg modification and Cys in deuterium oxide (D2O), use AgilentVNMRS600 600Hz 1H NMR instrument to measure 1H nuclear magnetic resonance spectrum, the number of scans is 256 times, its structure is as follows figure 1 shown.
[0056] Depend on figure 1 and 2 It can be seen that after sodium alginate is modified by cysteine, at 3284cm -1 ...
Embodiment 3-1
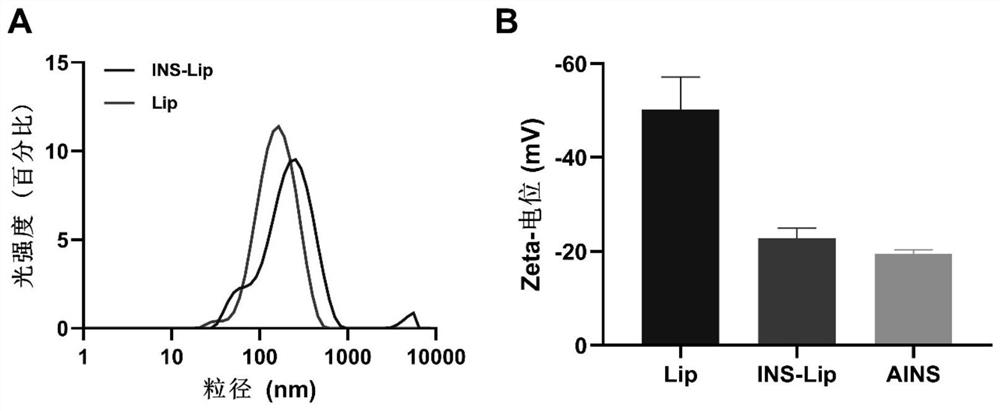
[0064] Preparation of insulin-loaded liposome hydrogels:
[0065] Step 1. Dissolve insulin in 10 mM HCl at a concentration of 2 mg / mL, and adjust the pH of the solution to 7.4 to obtain solution A; dissolve arginine in deionized water at a concentration of 6 mg / mL, and adjust the pH of the solution to 7.4 , to obtain solution B; filter solution A and solution B with 0.22 μm filter membrane respectively, and then mix the two solutions obtained after filtration according to the volume ratio of 1:1 to obtain insulin complex;
[0066] Step 2. Dissolve 10 mg of phosphatidylethanolamine and 3 mg of cholesterol in 10 mL of chloroform to obtain solution C, take 2 mL of chloroform and add 4 mL of insulin complex to obtain solution D; after solution C and solution D are mixed uniformly in a volume ratio of 1:1 , carried out rotary evaporation under reduced pressure, after the completion, the liposome solution was ultrasonicated for 2 minutes and squeezed with a 0.22 μm filter membrane f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com