Polyethylene glycol-based adhesive as well as preparation method and application thereof
A polyethylene glycol-based, multi-armed polyethylene glycol technology, applied in the field of polyethylene glycol-based adhesives and its preparation, can solve unsatisfactory sealing effects, unsatisfactory suturing technical requirements, nerve or spinal cord Secondary stenosis and other problems, to achieve the effect of not causing tissue nerve compression, good biodegradability, and preventing epidural fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] In the present invention, the preparation method of described phthalaldehyde-terminated multi-arm polyethylene glycol comprises the following steps:
[0045] A) react 1,3-dimethoxy-1,3-dihydroisobenzofuran-5-carboxylic acid succinimidyl ester with polyethylene glycol whose end group is amino to obtain a reaction product;
[0046] B) deprotecting the reaction product to obtain a polyethylene glycol derivative.
[0047] In the present invention, 1,3-dimethoxy-1,3-dihydroisobenzofuran-5-carboxylic acid succinimide ester and polyethylene glycol whose end group is an amino group are dissolved in an organic solvent, and the acid-binding The reaction is carried out in the presence of an agent, and sedimentation is carried out to obtain a reaction product.
[0048] The molar equivalent of the 1,3-dimethoxy-1,3-dihydroisobenzofuran-5-carboxylic acid succinimide ester is 1.2-5 of the amino group in the polyethylene glycol whose end group is an amino group. times, preferably 2 tim...
Embodiment 1
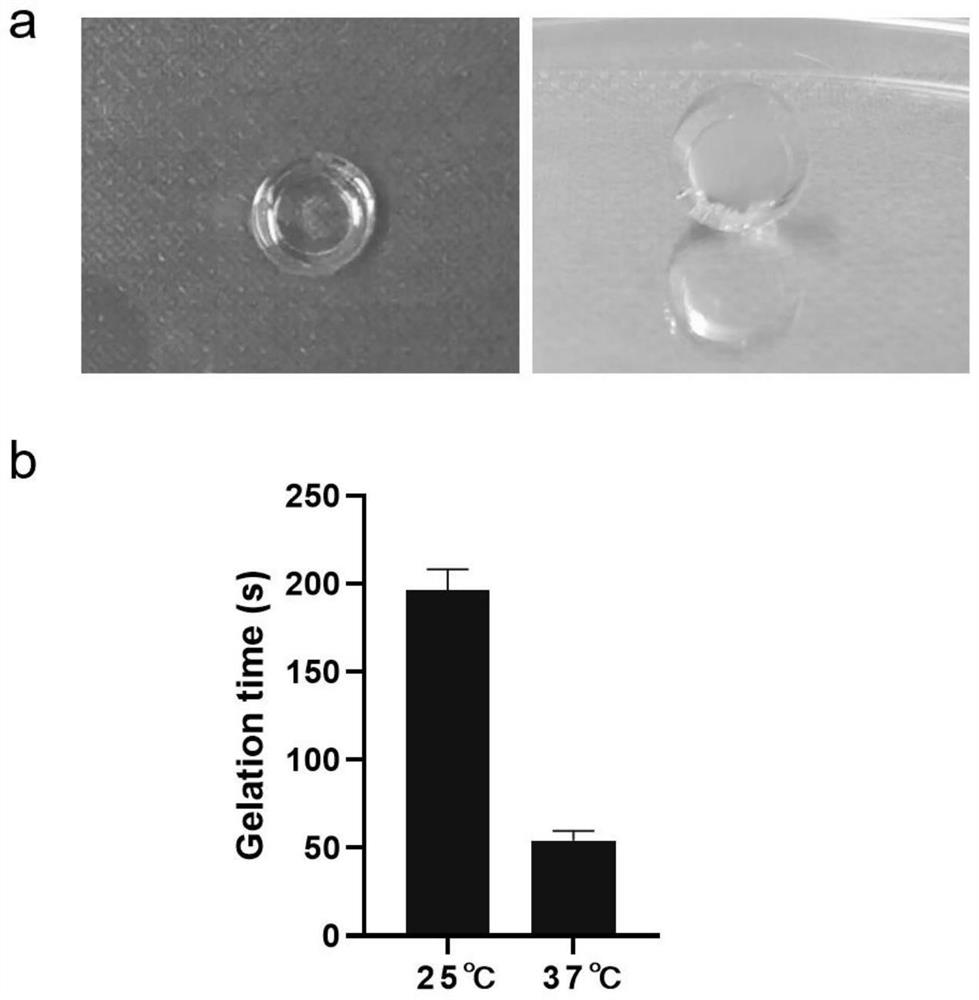
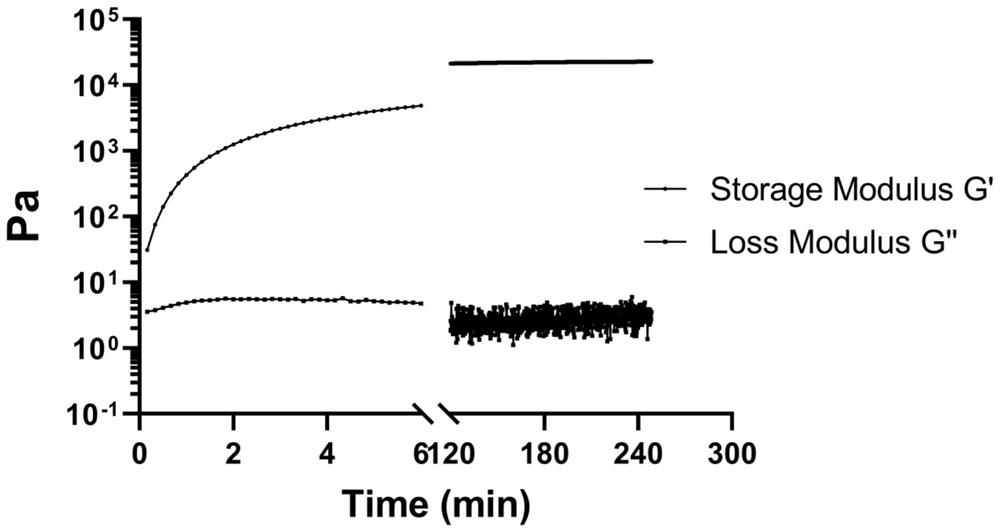
[0103] (1) Preparation, gelation state and gelation time of 4armPEG-OPA / Gelatin gel
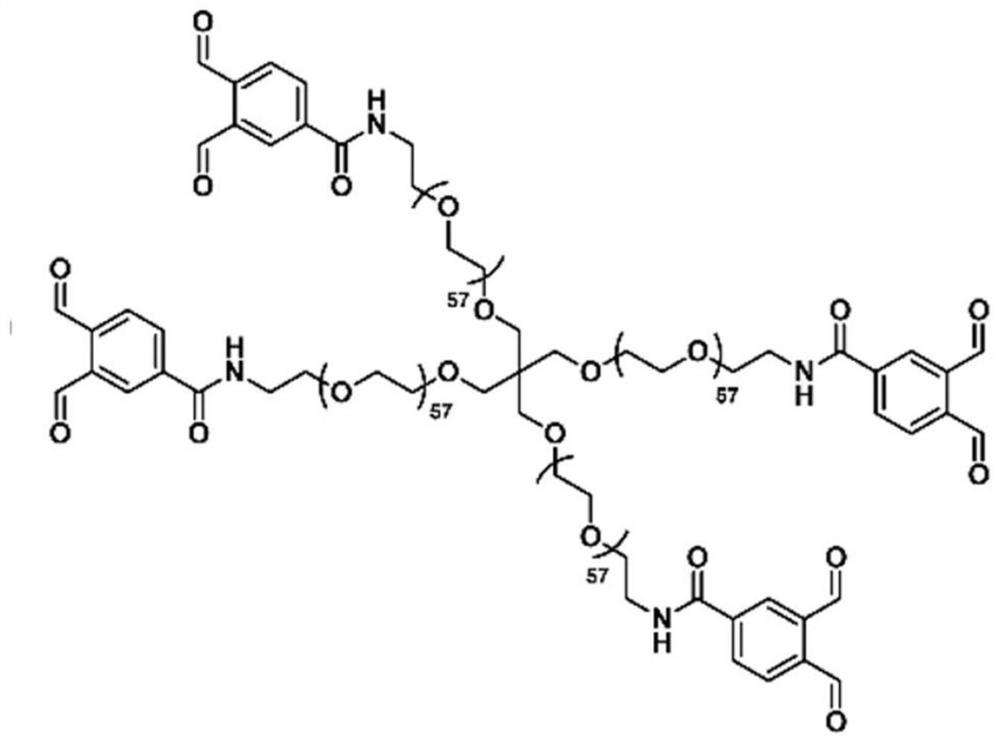
[0104] The hydrogel was obtained by cross-linking reaction of 4-arm polyethylene glycol (4armPEG-OPA) capped with phthalaldehyde and gelatin. The molecular formula of 4aPEG-OPA synthesized based on amino-terminated polyethylene glycol modification and succinimide active ester is as follows: figure 1 shown. The gel-forming mechanism is the use of two aldehyde groups in the para position and -NH 2 The cross-linking occurs through the acetal reaction to form a gel. First, porcine-derived gelatin was dialyzed with deionized water for 2-3 days, then lyophilized after removing impurities, and then the material was dissolved in PBS at 100 mg / ml. Since gelatin itself is a temperature-sensitive gel, the 10% (w / v) gelatin solution needs to be heated and dissolved in a 37-degree water bath in advance. When preparing a 10% 4aPEG-OPA solution, the solid powder should be taken out from -20°C, and then ...
Embodiment 2
[0114] Example 2: 4 armPEG-OPA / Gelatin hydrogel and fibrin glue repair rat dura mater
[0115]SD rats were selected and divided into 4aPEG-OPA / Gelatin group, fibrin glue group and blank group, with 4 rats in each group. Two time observation points were established: 1 week and 2 weeks. After successful anesthesia with pentobarbital sodium, the back skin was prepared and disinfected. The skin, subcutaneous tissue, and muscles were incised along the mid-lumbar segment of the back to expose the spinous process and lamina. The spinous process and lamina were removed with single-joint bone scissors, and the dural sac was exposed. The dural sac pulsation was visible under direct vision. The dura mater and the underlying arachnoid membrane were cut longitudinally with a micro-hook and micro-scissors, with a length of 1 cm. The clear fluid flow was seen to confirm the success of the modeling. Be careful not to damage the spinal cord during the modeling process. After successful mod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com