Organic compound and application thereof
A technology of organic compounds and chemical bonds, applied in the field of organic electroluminescent materials, to achieve the effects of improving luminous efficiency and stability, best luminous effect, and excellent hole transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0153] Synthesis Example 1: Organic Compound C1
[0154]
[0155] (1) Synthesis of intermediate C1-1
[0156] In a 1000mL single-necked flask equipped with magnetic stirring at room temperature, add 2,3-dichloronitrobenzene (30g, 156.25mmol), phenylboronic acid (47.63g, 390.64mmol), tris(dibenzylacetone)dipalladium ( 0)Pd 2 (dba) 3 (2.86g, 3.13mmol), 2-dicyclohexylphosphine-2',6'-dimethoxybiphenyl s-Phos (2.57g, 6.25mmol), potassium phosphate (66.33g, 312.51mmol), dioxygen 500 mL of hexacyclic ring, 50 mL of water, turn on stirring, replace nitrogen for 3 times, heat up to 100°C, and react overnight. As monitored by thin layer chromatography (TLC), the reaction of the starting material was complete. The reaction solution was cooled to room temperature, extracted with ethyl acetate, the supernatant liquid was taken, spin-dried, petroleum ether (PE):dichloromethane (DCM)=10:1 was passed through a 100-200 mesh silica gel column to obtain 40.56g of an oily liquid .
[015...
Synthetic example 2
[0162] Synthesis Example 2: Organic Compound C2
[0163]
[0164] (1) Synthesis of intermediate C2-2
[0165] Intermediate C1-2 (15 g, 61.14 mmol), 2-bromo-9,9-dimethylfluorene (16.70 g, 61.14 mmol), Pd 2 (dba) 3 (0.56g, 0.61mmol), 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride IPr.HCl (0.52g, 1.22mmol), NaOBu-t (11.75g, 122.29mmol) It was added to 500 mL of toluene, stirred evenly, and heated to 110° C. under nitrogen protection for overnight reaction. TLC monitoring showed that the reaction of the starting materials was complete. After cooling down, use a silica gel column to remove the solvent and remove 100 mL of methanol to precipitate a solid. The solid was collected, separated by column, PE:DCM=20:1, washed with methanol to obtain 12.85g of intermediate C2-2.
[0166] (2) Synthesis of organic compound C2
[0167] Intermediate C2-2 (12.85 g, 29.37 mmol), 2-bromo-9,9-diphenylfluorene (14 g, 35.24 mmol), Pd 2 (dba) 3 (0.54g, 0.59mmol), (t-Bu) 3 P (0.24 g, 1...
Synthetic example 3-14
[0170] The process route of Synthesis Example 3-14 is the same as that of Synthesis Example 1-2, the difference is that the raw materials used are different. The raw materials, target products and result characterization data are shown in Table 1; in Table 1, raw material A represents Raw material B indicates Raw material C means
[0171] Table 1
[0172]
[0173]
[0174]
[0175]
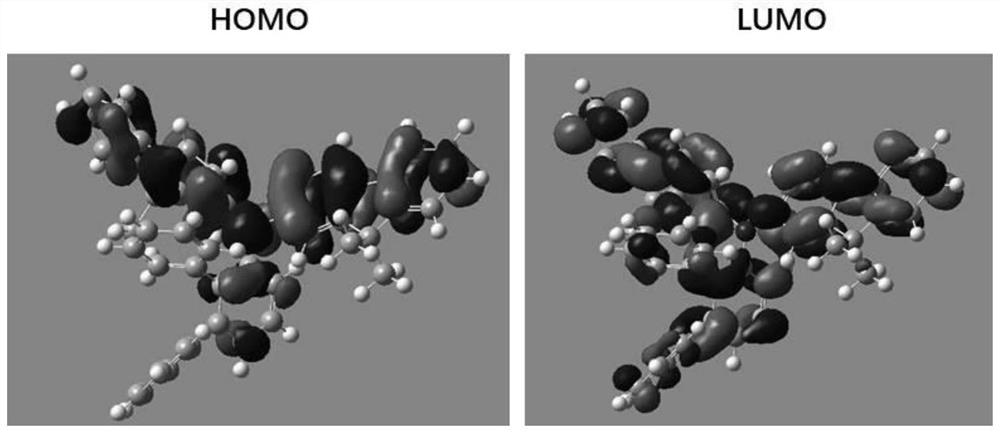
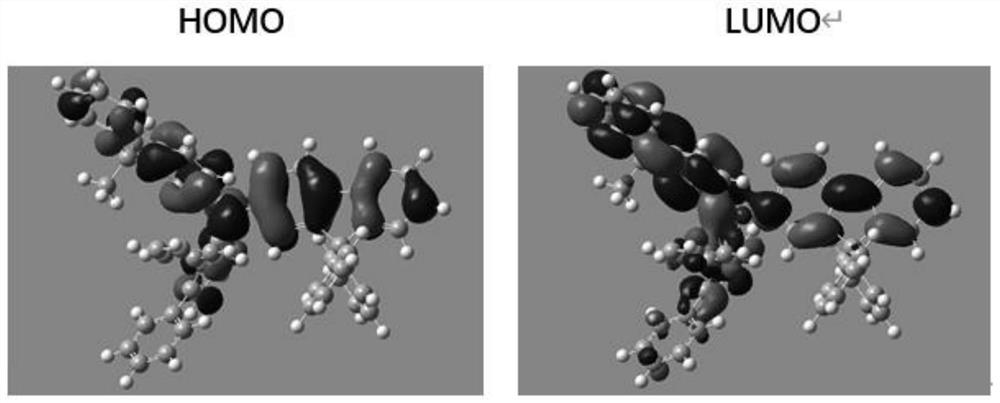
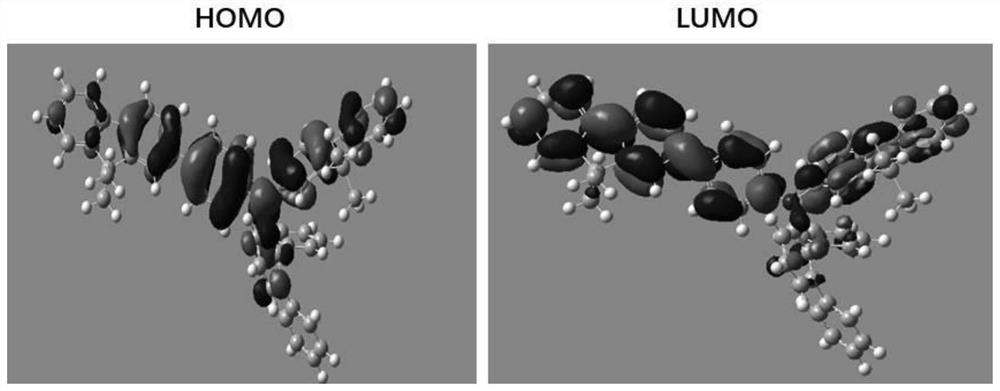
[0176] Quantum chemical calculations for compounds:
[0177] Among all the electron-filled orbitals of a compound, the orbital with the highest energy for the electron is called the highest occupied molecular orbital (HOMO). Conversely, among all the unfilled electron orbitals, the orbital with the lowest electron energy is called the lowest unoccupied molecular orbital (LUMO). The transport of charges in the material is the process by which electrons are continuously escaping from the HOMO and adding electrons to the LUMO. In quantum chemistry, the degree of overlap between HO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com