Anti-drug-resistance antibacterial amidine oligomer as well as preparation method and application thereof
A production method and oligomerization-like technology, which can be used in medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as high toxicity, and achieve low toxicity, broad-spectrum antibacterial activity, and high therapeutic index. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
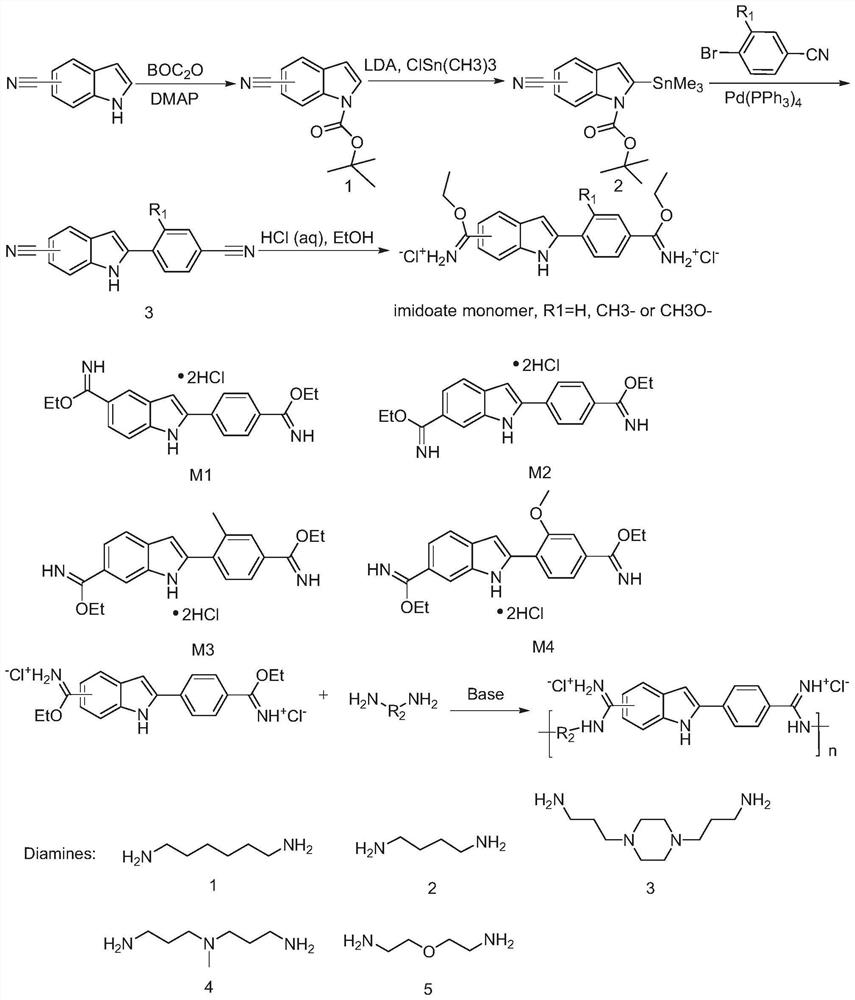
[0031] After testing, an antibacterial amidine oligomer with broad-spectrum antibacterial properties has the following chemical formula:
[0032]
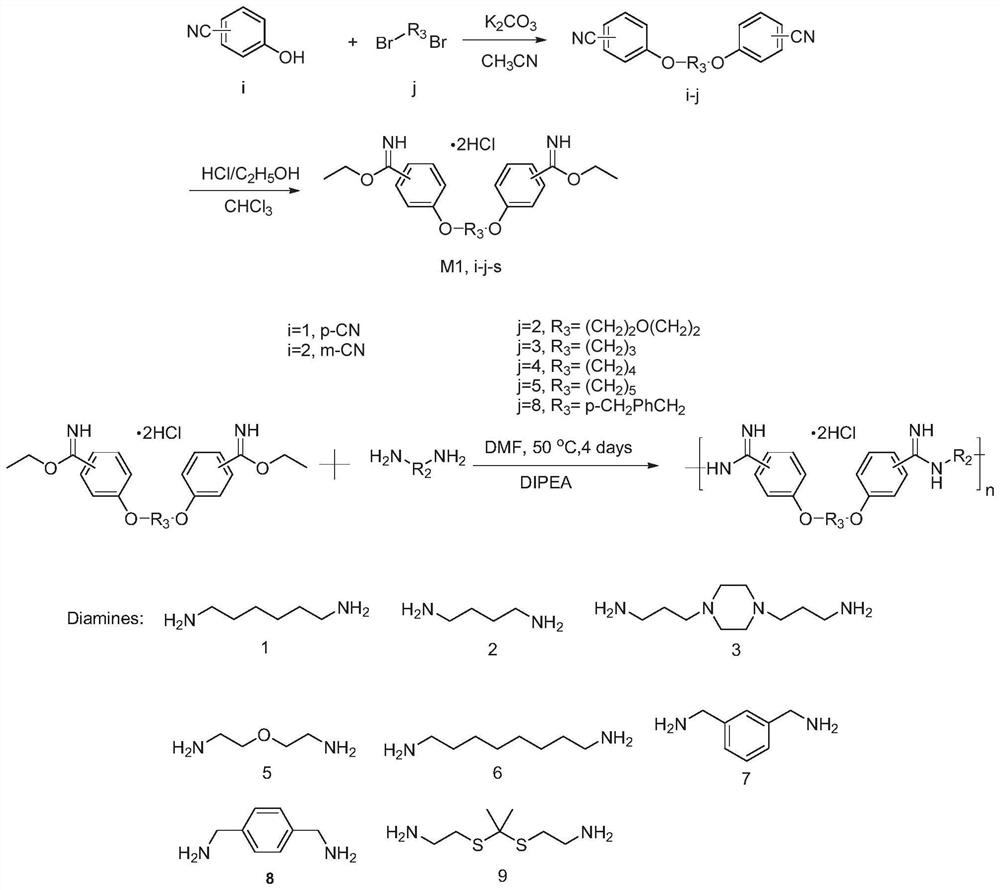
[0033] Here, 5≤n≤8, and R1 and R2 have the structures described above. It can be seen from the above structural formula that 81 compounds can be obtained by different combinations of R1 and R2. In order to facilitate the analysis and description of these compounds, 60 compounds are labeled in Table 1 below. It should be noted that, For those skilled in the art, unlabeled compounds can also be synthesized. The properties and synthesis methods of these unlabeled compounds are similar to those of the labeled 60 compounds, so they are not further analyzed and explained in the present invention.
[0034] The structural formulas of the 60 compounds, their antibacterial activities, and biocompatibility lists are shown in Table 1 and Table 2. From Table 2, it can be seen that the therapeutic indices of P1, P2, P3, P4, P6, and P7 are rel...
Embodiment 2
[0041] As shown in Figure 1, the present invention also discloses a method for synthesizing an antibacterial amidine oligomer with drug resistance, comprising the following steps:
[0042] S1: prepare a compound monomer having the following molecular formula:
[0043]
[0044] S2: react the compound monomer with H2N-R2-NH2, anhydrous N,N-dimethylformamide (DMF) and N,N-diisopropylethylamine (DIPEA) to obtain the amidines Oligomers, R1 and R2 have the structures described above.
[0045] Wherein, the above-mentioned step S1 includes the following steps:
[0046] S11: Weigh 5-cyanoindole or 6-cyanoindole and di-tert-butyl dicarbonate, then add dichloromethane and mix evenly, add a catalytic amount of 4-dimethylaminopyridine (DMAP) at 2-6 After stirring at ℃ for half an hour, it was returned to room temperature of 20-25 ℃ and stirred overnight, the mixture was filtered and washed, and then spin-dried with a rotary evaporator to obtain a solid product, and then the product wa...
experiment example 1
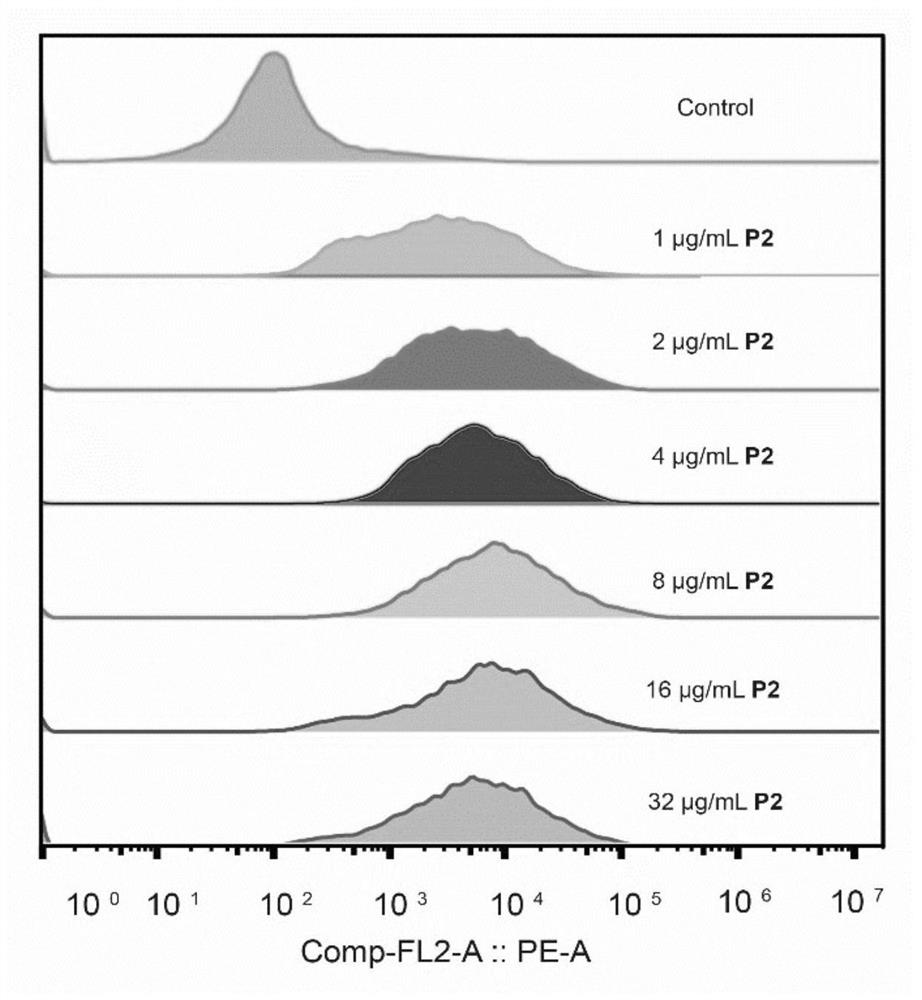
[0053] This experimental example is described in detail with the P2 compound:
[0054] Materials and methods: All reagents were provided by TCI (USA), Sigma-Aldrich, McLean, Adamas and other organic reagent companies and Biyuntian Biotechnology Company, and were used directly without further purification (unless otherwise noted). The ultrapure water used in the experiment was obtained from the Milli-Q purification instrument. The inert gas is nitrogen.
[0055] The synthesis steps of P2 are shown in Figure 1. First, the synthesis of 4',6-diamidino-2-phenylindole hydrochloride monomer was carried out. Weigh 6-cyanoindole (10 g, 70.3 mmol) and di-tert-butyl dicarbonate (BOC) (35.36 g, 162 mmol), then add 100 mL of dichloromethane and mix well, then add a catalytic amount of 4-dimethylamino Pyridine (DMAP) (0.86 g, 7.03 mmol) was stirred at room temperature for 12-24 h to obtain a mixture. The mixture was extracted and spin-dried to obtain a solid mixture. The solid mixture wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com