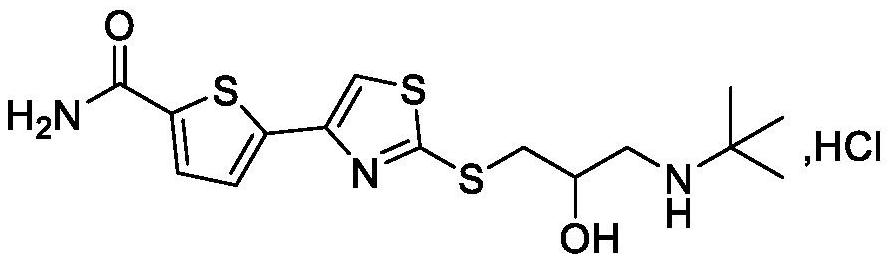
Preparation process of arotinolol hydrochloride intermediate
A preparation technology of alololol hydrochloride, which is applied in the field of medicinal chemical preparation, can solve the problems of high-temperature reaction, large equipment corrosion, and many impurities, and achieve the effect of less synthesis steps, high atom utilization rate, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
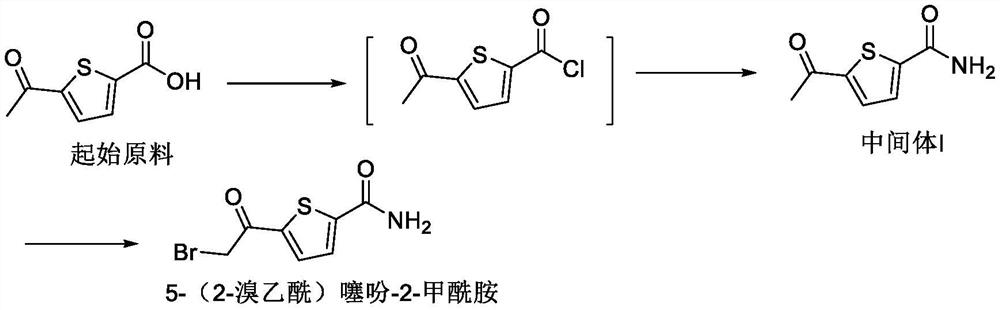
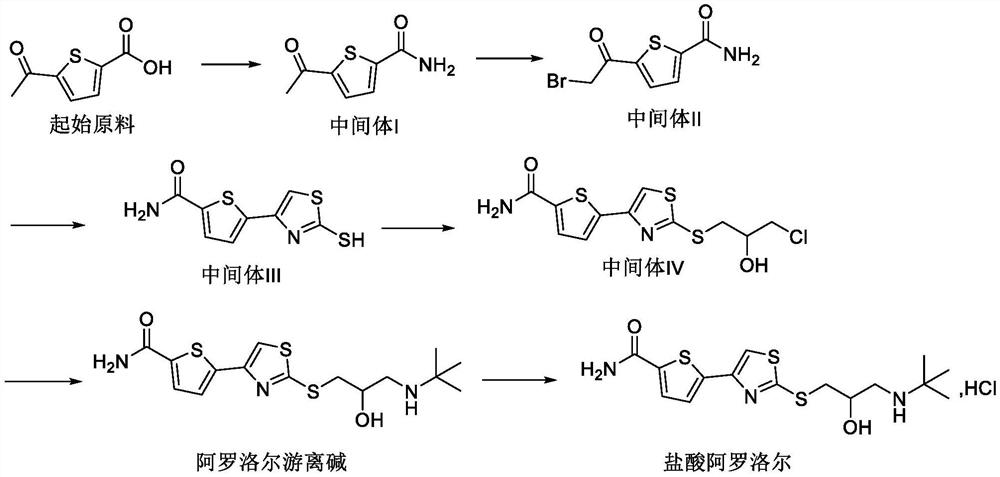
[0042] Example 1: Preparation of 5-(2-bromoacetyl)thiophene-2-carboxamide
[0043] (a) Preparation of Intermediate I:
[0044] Suspend 11.5kg of 5-acetyl-thiophene-2-carboxylic acid in 152.4kg of dichloromethane, add 230g of N,N-dimethylformamide, replace with nitrogen, stir and cool down to 0°C, and slowly add 11.15kg of oxalyl chloride. After the addition was completed, the temperature was slowly raised to 15-25°C and stirred. After the reaction was completed, the reaction solution was concentrated to dryness under reduced pressure. 152.4 kg of dichloromethane was added to the residue, and after stirring and dissolving completely, it was used for later use. 23L of concentrated ammonia water was added to the reaction kettle, cooled to -5~5°C, and the dichloromethane solution of the above-mentioned intermediate state—acyl chloride was slowly added. After the addition is completed, centrifugation, and the filter cake is rinsed with water. After drying under reduced pressure...
Embodiment 2
[0049] Example 2: Preparation of 5-(2-bromoacetyl)thiophene-2-carboxamide
[0050] (a) Preparation of Intermediate I:
[0051]115g of 5-acetyl-thiophene-2-carboxylic acid was suspended in 1524g of acetonitrile, 2.3g of N,N-dimethylformamide was added, and after nitrogen replacement, the temperature was lowered to 0°C with stirring, and 128.9g of oxalyl chloride was slowly added. After the addition was completed, the temperature was slowly raised to 30-40°C and stirred. After the reaction was completed, the reaction solution was concentrated to dryness under reduced pressure. 1524 g of acetonitrile was added to the residue, and after stirring to dissolve completely, it was used for later use. 1.15L of concentrated ammonia water was added to the reaction kettle, the temperature was lowered to 15-25°C, and the acetonitrile solution of the above-mentioned intermediate state-acyl chloride was slowly added. After adding, filter and rinse the filter cake with water. After drying ...
Embodiment 3
[0054] Example 3: Preparation of 5-(2-bromoacetyl)thiophene-2-carboxamide
[0055] (a) Preparation of Intermediate I:
[0056] 115g of 5-acetyl-thiophene-2-carboxylic acid was suspended in 1524g of tetrahydrofuran, 2.3g of N,N-dimethylformamide was added, and after nitrogen replacement, the temperature was lowered to 0°C with stirring, and 94.4g of oxalyl chloride was slowly added. After the addition was completed, the temperature was slowly raised to 10-15°C and stirred. After the reaction was completed, the reaction solution was concentrated to dryness under reduced pressure. 1524 g of tetrahydrofuran was added to the residue, and the mixture was stirred and dissolved, and it was used for later use. 172.5ml of concentrated ammonia water was added to the reaction kettle, the temperature was lowered to -10~0°C, and the tetrahydrofuran solution of the above-mentioned intermediate state-acyl chloride was slowly added. After adding, filter and rinse the filter cake with water....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com