Method for testing efficacy of porcine circovirus type 2 inactivated vaccine
A technology of vaccine and effectiveness, which is applied in the field of testing the effectiveness of porcine circovirus type 2 inactivated vaccines, can solve the problem of not truly reflecting the immune effect of porcine circovirus type 2 inactivated vaccines, large differences between batches of test results, and problems in testing Pig screening time-consuming and other issues, to achieve the effect of short time-consuming, high stability, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of porcine circovirus type 2 cytotoxicity is as follows:
[0035] Select the well-grown Pk15 cell monolayer, pour off the cell fluid, add the virus seed according to the inoculation amount of 5% (V / V), add the virus culture solution containing 2% newborn bovine serum and 3mmol of D-glucosamine salt, Incubate at 37°C. Harvest 72 hours after exposure, freeze and thaw for three times, centrifuge at 3000 r / min for 20 min, take the supernatant, and quantitatively divide it as porcine circovirus type 2 cytotoxicity.
[0036] The preparation method of PCV2 inactivated antigen liquid is as follows:
[0037] Add β-propiolactone (purity 97%) according to 1 / 4000 (V / V) of the total amount of virus solution, stir with addition, shake fully, inactivate at 4°C for 24 hours, and shake once every 4 hours. The inactivated virus liquid was hydrolyzed at 37° C. for 2 hours, and stored at 2-8° C. The inactivation test was qualified as PCV2 inactivated antigen liquid...
Embodiment 1
[0038] Embodiment 1, the method that examines porcine circovirus type 2 inactivated vaccine efficacy
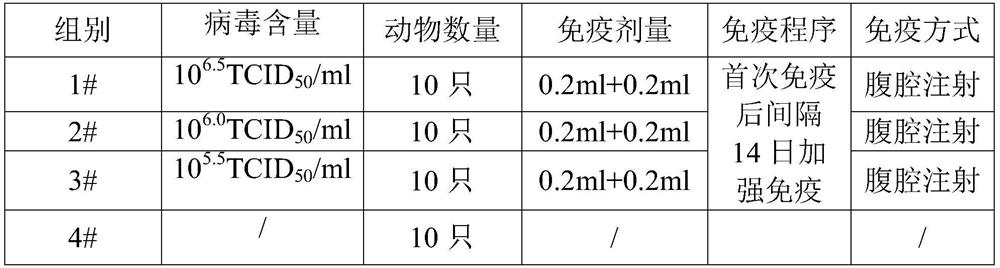
[0039] 1 Vaccine preparation Dilute the PCV2 inactivated antigen solution with PBS to 10 6.5 TCID 50 / ml, 10 6.0 TCID 50 / ml and 10 5.5 TCID 50 / ml emulsified with adjuvant to prepare vaccines with different antigen contents for later use.
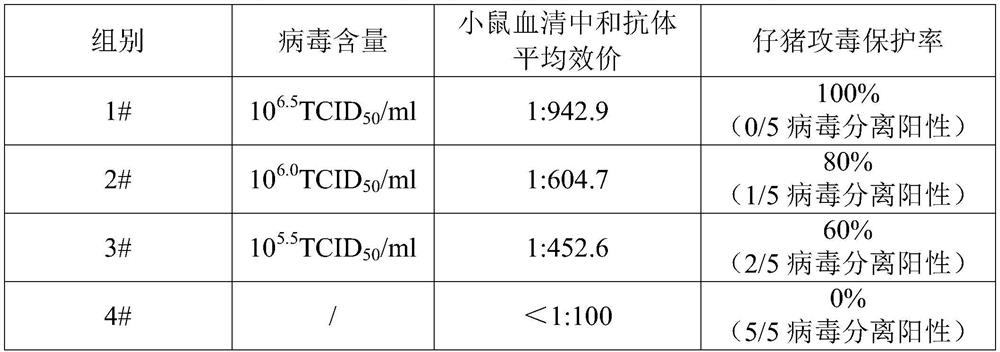
[0040] 2. Immunization of mice 40 Balb / c female mice were randomly divided into 4 groups, 10 mice in each group, immunized with PCV2 inactivated vaccine, each group was intraperitoneally injected with 0.2ml, and the immunization was boosted once with the same dose and the same inoculation method after 14 days. A non-immunized control group was established, and the immunization information is shown in Table 1. The sera of mice were collected 21 days after booster immunization for neutralizing antibody titer determination.
[0041] Table 1. Vaccine Immunization Information
[0042]
[0043] 3 Serum neutralizing antibody detection...
experiment example 1
[0065] Experimental example 1. Screening of judgment criteria for serum neutralizing antibody detection
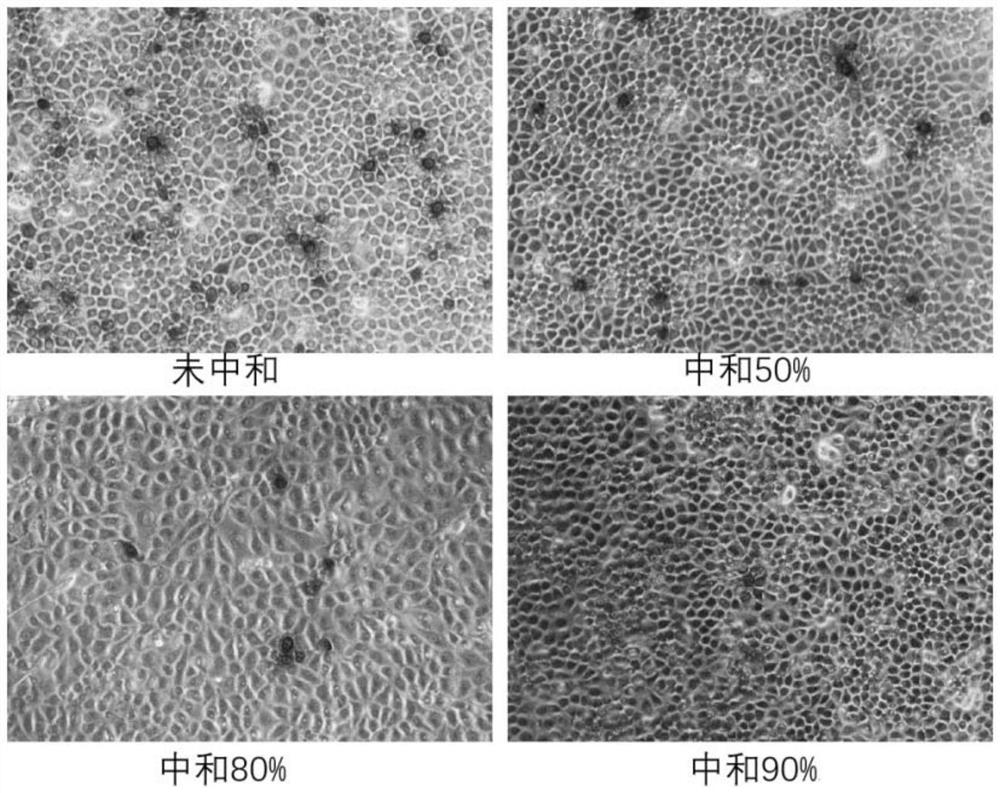
[0066] (1) In addition, collect the mouse serum on the 21st day after the vaccine booster immunization in Example 1 for PCV2 neutralizing antibody titer determination. During the determination, steps 3.1-3.8 are the same as those in Example 1, but the determination standard in step 3.9 is: sample The number of PCV2-positive cells in the neutralization wells decreased by 80% compared with the number of positive cells in the virus control wells, and the neutralization efficacy was judged.
[0067] The serum neutralizing antibody levels of mice were detected under the above criteria, and the results were as follows:
[0068] The antibody levels of 10 mice in group 1# were 1:1084, 1:1331, 1:1184, 1:1347, 1:1184, 1:928, 1:994, 1:1255, 1:891, 1:1331 ; Antibody levels of 10 mice in 2# group were: 1:762, 1:600, 1:872, 1:502, 1:891, 1:994, 1:762, 1:802, 1:868, 1 :994;;The antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com