Synthesis method of near-infrared two-region fluorescent dye FD-1080
A synthesis method and technology of fluorescent dyes, applied in the direction of organic dyes, luminescent materials, chemical instruments and methods, etc., can solve the problems of high production cost and high price of FD-1080, and achieve the effect of increasing yield and simple treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
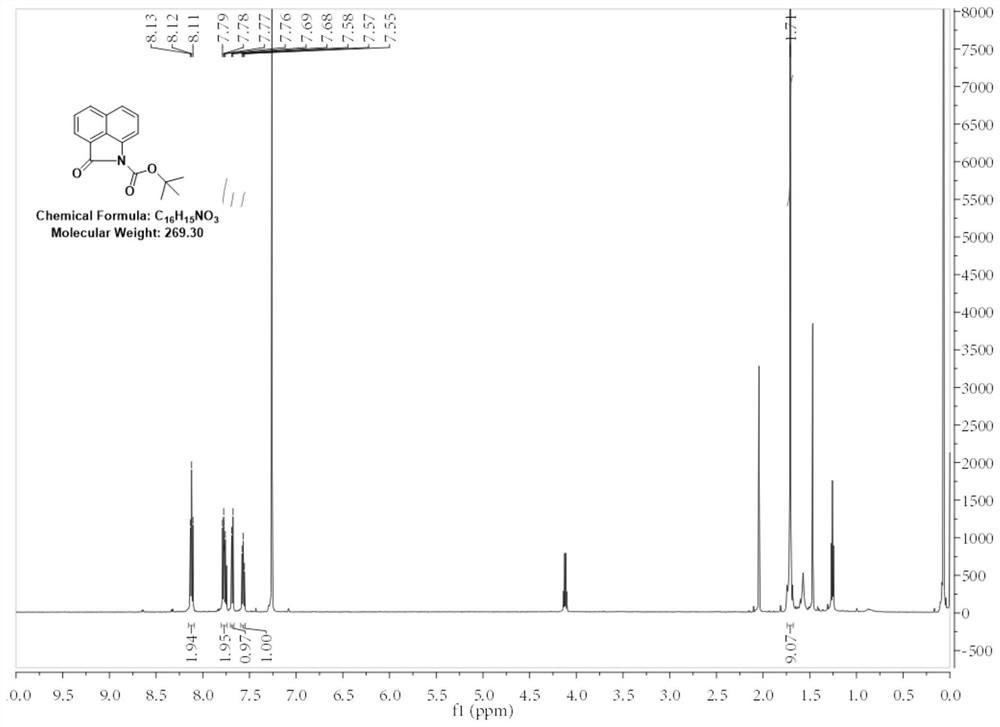
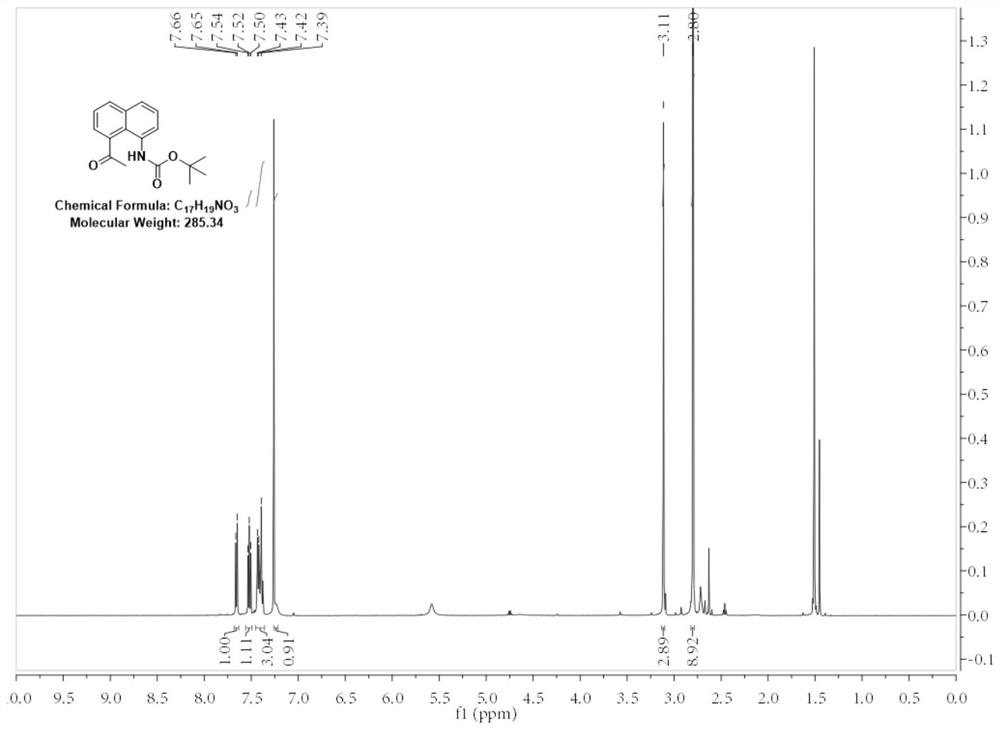
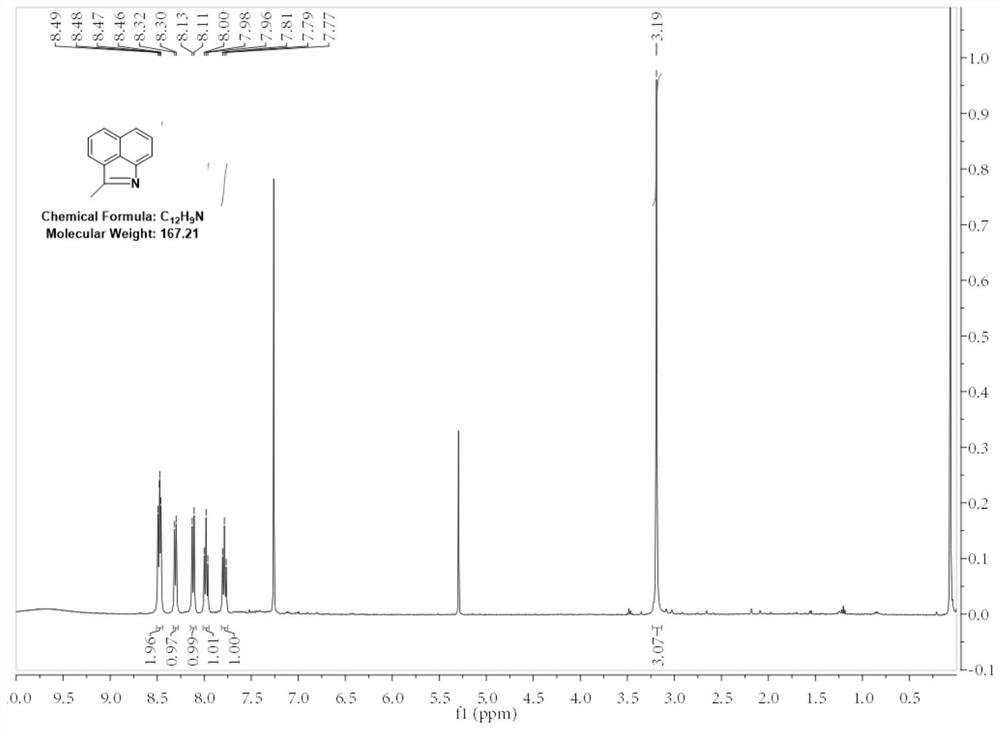
[0040] 1. Add 10g (0.059mol) of benzo[cd]indol-2(1H)-one to a 500mL round-bottomed flask, then add 100mL of acetonitrile and stir until benzo[cd]indol-2(1H)-one All dissolved, then 10.83g (0.088mol) 4-dimethylaminopyridine was added, and finally 19.35g (0.088mol) di-tert-butyl dicarbonate was added to the round-bottomed flask in batches under stirring. A large number of bubbles were generated but there was no obvious heating phenomenon, and the bottle was a turbid liquid. After adding the material, the solid in the bottle was ultrasonicated for 2 minutes to make it evenly dispersed, and the reaction was stirred at room temperature for 2 hours. First, the acetonitrile was distilled off under reduced pressure, and then 0.5mol / L hydrochloric acid was added. The unreacted 4-dimethylaminopyridine was washed away, and finally the mixture was extracted twice with ethyl acetate. The organic phase was dried with anhydrous sodium sulfate and concentrated. After the concentration was comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com