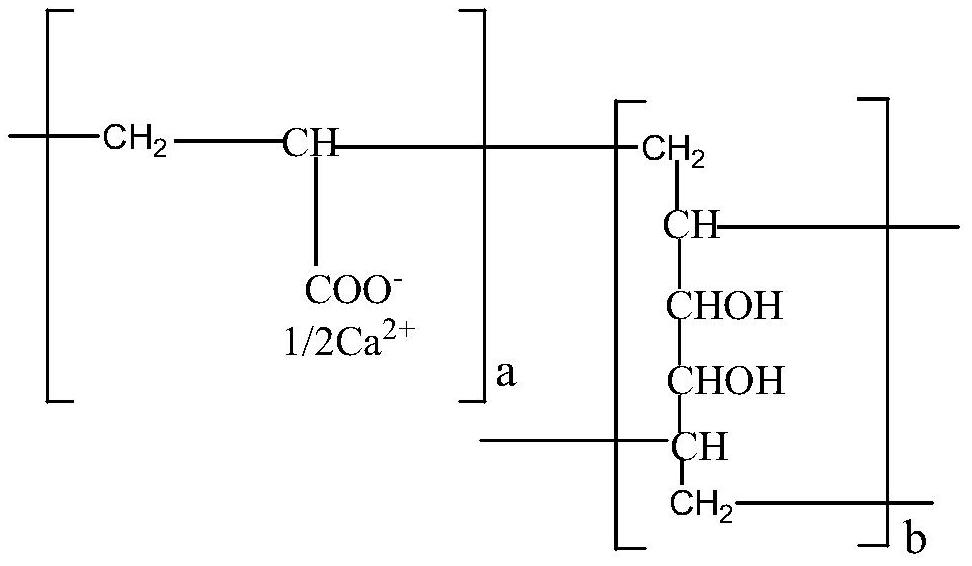
Calcium polycarbophil tablet easy to rapidly and completely disintegrate
A technology of polycarbophil calcium tablets and polycarbophil calcium, which is applied in the field of pharmaceutical preparations, can solve problems such as complex processes and unfavorable labor protection, and achieve the effects of simple preparation process, accelerated drug dissolution, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
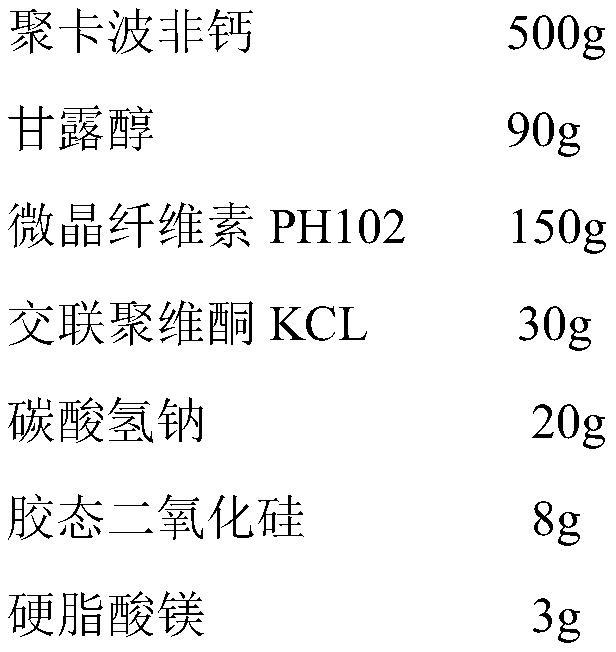
[0034] 1) Prescription
[0035]
[0036] 2) Preparation process
[0037] (a) Put calcium polycarbophil and mannitol into the lifting hopper mixer in turn according to the recipe quantity, and mix at 10rpm / min for 15min.
[0038] (b) Keep the air supply pressure of the jet mill at 0.6-0.8Mpa, feed the mixed powder of polycarbophil calcium and mannitol to the pulverizer, carry out jet milling, and co-process the polycarbophil calcium and mannitol after jet milling The particle size of the material is controlled in the range of 50-100 μm.
[0039] (c) The polycarbophil calcium and mannitol co-processing product of recipe quantity, microcrystalline cellulose, crospovidone, sodium bicarbonate and colloidal silicon dioxide are successively put into the lifting hopper mixer, and mixed for 30min.
[0040] (d) granulating the above-mentioned mixed powder through a conical granulator, the speed of the granulator is 1000 rpm, and the aperture of the screen mesh is 2 mm.
[0041] (e...
Embodiment 2
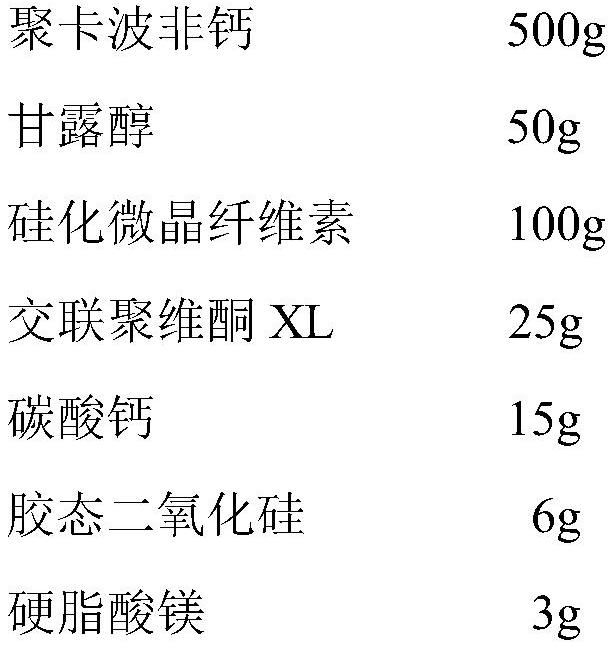
[0044] 1) Prescription
[0045]
[0046] 2) Preparation process
[0047] (a) Put calcium polycarbophil and mannitol into the lifting hopper mixer in turn according to the recipe quantity, and mix at 10rpm / min for 15min.
[0048] (b) Keep the air supply pressure of the jet mill at 0.6-0.8Mpa, feed the mixed powder of polycarbophil calcium and mannitol to the pulverizer, carry out jet milling, and co-process the polycarbophil calcium and mannitol after jet milling The particle size of the material is controlled in the range of 50-100 μm.
[0049] (c) The polycarbophil calcium and mannitol co-treated product of recipe quantity, silicified microcrystalline cellulose, crospovidone, calcium carbonate and colloidal silicon dioxide are successively put into the lifting hopper mixer, and mixed for 30min.
[0050] (d) granulating the above-mentioned mixed powder through a conical granulator, the speed of the granulator is 1000 rpm, and the aperture of the screen mesh is 2 mm.
[0...
Embodiment 3
[0054] 1) Prescription
[0055]
[0056] 2) Preparation process
[0057] (a) Put calcium polycarbophil and mannitol into the lifting hopper mixer in turn according to the recipe quantity, and mix at 10rpm / min for 15min.
[0058] (b) Keep the air supply pressure of the jet mill at 0.6-0.8Mpa, feed the mixed powder of polycarbophil calcium and mannitol to the pulverizer, carry out jet milling, and co-process the polycarbophil calcium and mannitol after jet milling The particle size of the material is controlled in the range of 50-100 μm.
[0059] (c) the polycarbophil calcium of recipe quantity and mannitol co-treated product, cellulose lactose, croscarmellose sodium, sodium carbonate and colloidal silicon dioxide are dropped into the lifting hopper mixer successively, mixed for 30min .
[0060] (d) granulating the above-mentioned mixed powder through a conical granulator, the speed of the granulator is 1000 rpm, and the aperture of the screen mesh is 2 mm.
[0061] (e) t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com