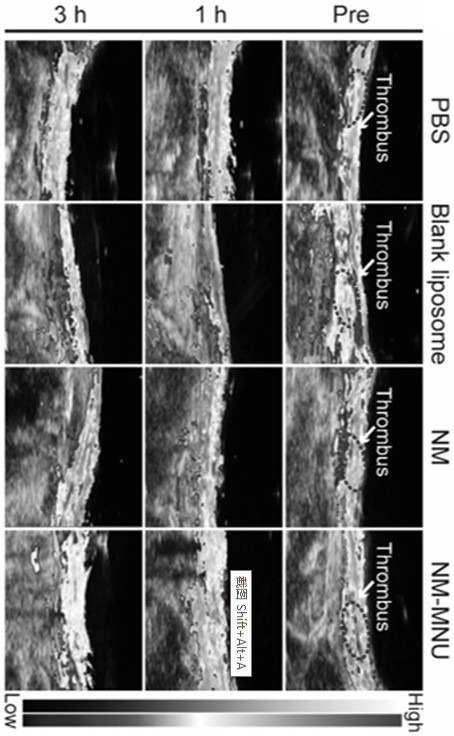
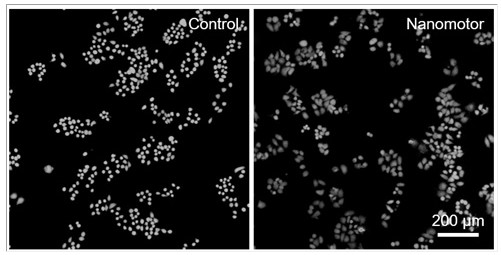
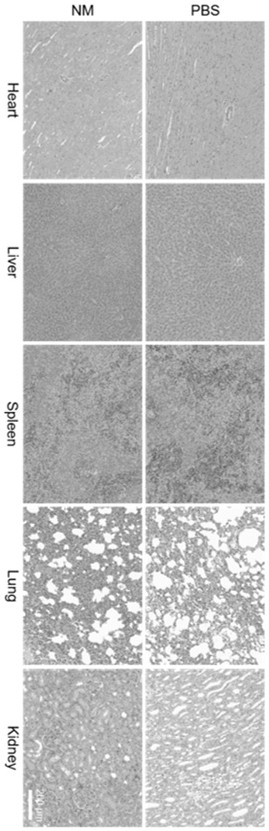
Ultrasonic and near-infrared light excited phase-change magnetic nano thrombolytic drug and preparation method thereof
A near-infrared light, magnetic nanotechnology, applied in the fields of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of lack of targeting, unable to quickly clear large thrombus at a fixed point, etc., and achieve long retention time, excellent photothermal effect, The effect of improving the efficiency of thrombolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention discloses a phase-change magnetic nano-thrombotic drug excited by ultrasound and near-infrared light and a preparation method thereof, which mainly include the following steps:
[0033] (1) The nanoscale Fe 3 O 4 Dispersed in methanol and pretreated with ultrasonic;
[0034] (2) Dissolve DPPC, DPPA, DPPE and DSPE-mPEG-2000 in chloroform;
[0035] (3) Nanoscale Fe dispersed in methanol obtained in step (1) 3 O 4 adding into the chloroform solution obtained in step (2), and evaporating the solvent with a rotary evaporator to form a uniform lipid film on the inner surface of the rotary evaporator;
[0036] (4) Prepare a hydration solution, then add the hydration solution into the rotary evaporation bottle in step (3), and ultrasonically peel off the lipid film and disperse it in the hydration solution;
[0037] (5) The hydration solution with dispersed lipid film obtained in step (4) is crushed with a cell crusher, and PFH liquid is added dropwise during...
Embodiment 1
[0043] (1) 10 mg Fe 3 O 4 Disperse in 5 mL methanol and sonicate for 20 min.
[0044] (2) DPPC, DPPA, DPPE, DSPE-mPEG-2000 were weighed and dissolved in 10 mL of chloroform, respectively weighing 20 mg: 6 mg: 6 mg: 6 mg.
[0045] (3) Nanoscale Fe dispersed in methanol obtained in step (1) 3 O 4 It is added to the chloroform solution obtained in step (2), and the solvent is evaporated to dryness with a rotary evaporator to form a uniform lipid film on the inner surface of the rotary evaporator. The rotary evaporation operation was performed with a temperature of 55 °C, a vacuum of 200 bar, a rotation speed of 300 rpm, and a rotary evaporation time of 30 min.
[0046] (4) The hydration solution was prepared, and the components of the hydration solution were PBS buffer solution, glycerol and block polyether F-68 and UK. The formulated quantities were 5 mL, 0.5 mL, 3 mg, and 20 mg, respectively. Then add the hydration solution into the rotary evaporation bottle in step (3), ...
Embodiment 2
[0051] (1) 5 mg Fe 3 O 4 Disperse in 5 mL methanol and sonicate for 20 min.
[0052] (2) DPPC, DPPA, DPPE, DSPE-mPEG-2000 were weighed and dissolved in 10 mL of chloroform, respectively weighing 20 mg: 6 mg: 6 mg: 6 mg.
[0053] (3) Nanoscale Fe dispersed in methanol obtained in step (1) 3 O 4 It is added to the chloroform solution obtained in step (2), and the solvent is evaporated to dryness with a rotary evaporator to form a uniform lipid film on the inner surface of the rotary evaporator. The rotary evaporation operation was performed with a temperature of 55 °C, a vacuum of 200 bar, a rotation speed of 300 rpm, and a rotary evaporation time of 30 min.
[0054] (4) The hydration solution was prepared, and the components of the hydration solution were PBS buffer solution, glycerol and block polyether F-68 and UK. The formulated quantities were 5 mL, 0.5 mL, 3 mg, and 20 mg, respectively. Then add the hydration solution into the rotary evaporation bottle in step (3), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com