Cyanoacrylate medical adhesive as well as preparation method and application thereof
A technology of cyanoacrylate and cyanoacrylic acid, which is applied in the fields of application, medical science, surgery, etc., can solve the problems affecting the closure rate, deglue phenomenon, easy generation of debris, etc., and achieve the improvement of tensile strength and closure strength, Excellent bonding performance, the effect of improving comfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
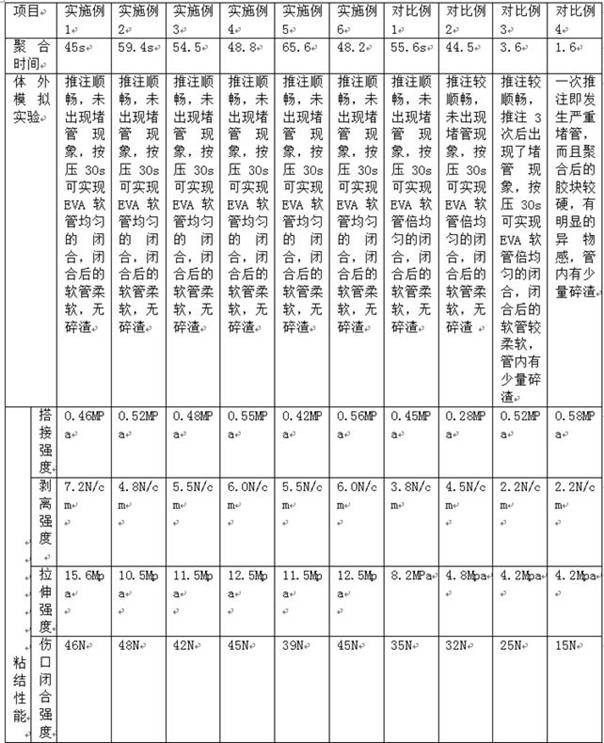
[0063] 1) Preparation of samples to be tested:
[0064] Select the pig skin on both sides of the pig's abdomen, remove the fat layer on the surface until the dermis is exposed, wash and dry, and cut into a rectangle. The length is greater than 5 cm, the width is 2.5 ± 0.1 cm, and the thickness is less than 5 mm.
[0065] 2) Bonding performance test: Use medical glue to bond two pieces of pig skins together, and test the bonding performance indicators of the gel hemostatic material according to the testing method in YY / T 0729-2009.
[0066] 4. Rockwell hardness test
[0067] Take a 20 cm EVA tube with a diameter of 1 cm, and after injecting sheep blood, close one end and flatten it to a sheet with a thickness of about 2 mm. Use a 4 Fr PTFE tube to inject medical glue into the EVA tube. At the same time, withdraw the PTFE tube until 2 mL of medical glue is completely injected into the EVA tube. After complete polymerization, use a scalpel to cut off the tube, rinse with purif...
preparation example 1
[0074] Preparation Example 1: Preparation of n-butyl cyanoacrylate
[0075] Add n-butyl cyanoacetate (200 g, 1.42 mol, Tixiai (Shanghai) Chemical Industry Development Co., Ltd.), 1,2-dichloroethane (100 ml, Sinopharm Chemical Reagent Co., Ltd.) to 1 L of In a four-necked flask, heated to between 65-70 ℃, add dropwise aqueous formaldehyde solution (36%, 109 g, 1.31 mol, Sinopharm Chemical Reagent Co., Ltd.), piperidine (1 mL, Sinopharm Chemical Reagent Co., Ltd.) During the dropwise addition, the reaction temperature was controlled not to exceed 80°C. After the dropwise addition, heat to reflux, and continue to stir for 3 hours, stop heating, add 10 mL of phosphoric acid, and stir for 10 min, install a water separation device to separate water, until no obvious water droplets can be seen in the system, change the separation. The water device is a distillation device to remove the residual solvent in the system, and add dibutyl phthalate (80mL, Sinopharm Chemical Reagent Co.,...
preparation example 2
[0077] Preparation Example 2: Preparation of sec-octyl cyanoacrylate
[0078] According to the method similar to Preparation Example 1, prepare sec-octyl cyanoacrylate, feed intake and yield are as follows: sec-octyl cyanoacetate (200 g, 1.01 mol, Tixiai (Shanghai) Chemical Industry Development Co., Ltd.), 1 ,2-dichloroethane (200ml, Sinopharm Chemical Reagent Co., Ltd.), aqueous formaldehyde solution (36%, 78 g, 0.94 mol, Sinopharm Group Chemical Reagent Co., Ltd.), piperidine (1 mL, Sinopharm Group Chemical Reagent Co., Ltd.) ). Dibutyl phthalate (80 mL, Sinopharm Group Chemical Reagent Co., Ltd.), hydroquinone (4 g, Sinopharm Group Chemical Reagent Co., Ltd.), p-toluenesulfonic acid (2 g, Sinopharm Group Chemical Reagent Co., Ltd.) , phosphorus pentoxide (4 g, Sinopharm Chemical Reagent Co., Ltd.) to obtain pure sec-octyl cyanoacrylate (102 g, 0.49 mol, yield 48.5%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com