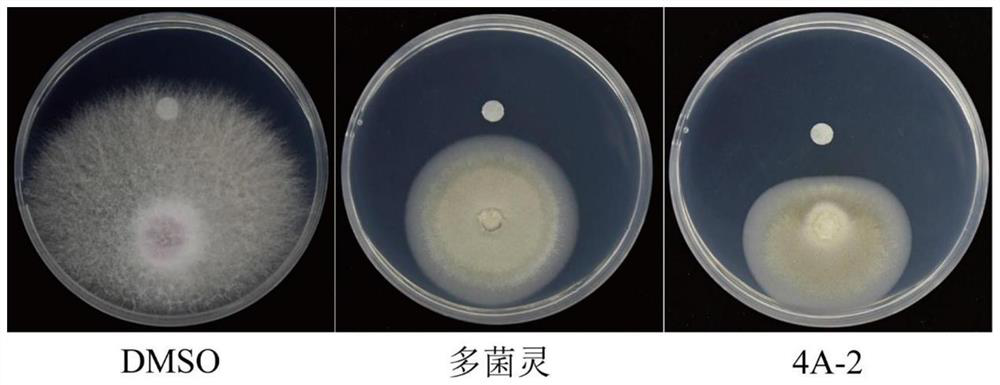
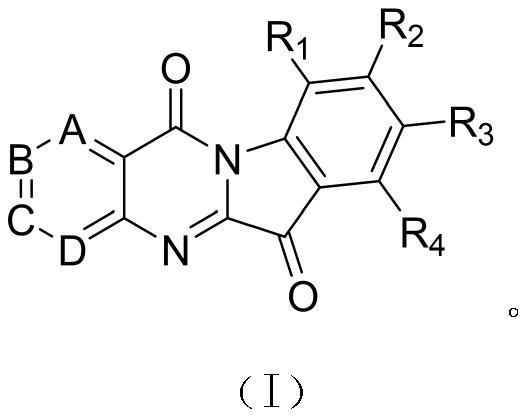
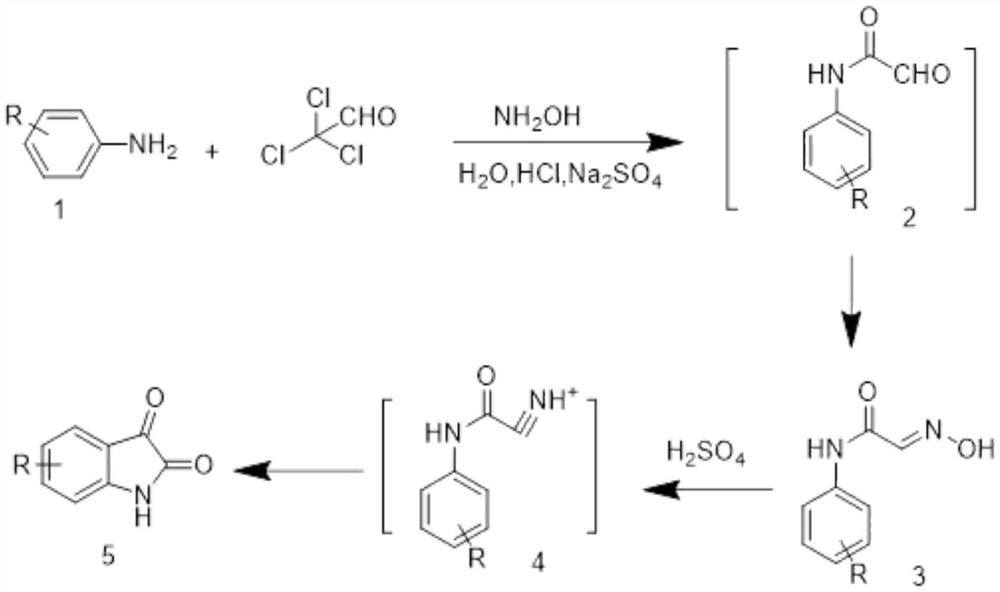
Synthesis of azacytanthrin derivative and application of azacytanthrin derivative in bactericide for preventing and treating phytopathogen bacteria and fungi and plant virus-resistant preparation
A technology of plant pathogenic bacteria and heterotryptanthin, which is applied in the field of medicinal chemistry and pesticide application, and can solve the problems of high toxicity and reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1 Compound 4A-1 (pyrido[2',3':4,5]pyrimido[1,2-a]indol-5(11H)-one)
[0092] 2-Aminonicotinic acid (0.01 mol) was added to 25 mL of dry dichloromethane, thionyl chloride (0.05 mL) was added to reflux for 3 h, the solvent was spin-dried, chloroform was added quickly, and isatin (0.01 mol) was added In the reaction system, a catalytic equivalent of triethylamine (0.001 mol) was added dropwise, refluxed for 2 hours, monitored by TLC, and when the reaction was complete, the solvent was spin-dried and separated by column chromatography (dichloromethane / methanol was 40:1 elution) , the target compound 4A-1 was obtained.
[0093]
[0094] Orange-yellow solid, yield 65%. M.P.>300°C, 1H NMR (500MHz, DMSO-d6) δ 9.04 (dd, J=4.6, 2.2Hz, 1H), 8.67 (dd, J=7.8, 2.0Hz, 1H), 8.41(d, J=8.0Hz, 1H), 7.88(d, J=7.4Hz, 2H), 7.71(dd, J=7.8, 4.6Hz, 1H), 7.47(t, J=7.4Hz, 1H).
[0095] 13C NMR (101MHz, DMSO) δ182.76, 158.58, 157.70, 156.58, 148.08, 146.21, 138.42, 136.79, 127.75, 125...
Embodiment 2
[0097] Example 2 Compound 4A-2 (9-fluoropyrido[2',3':4,5]pyrimido[1,2-a]indol-5(11H)-one)
[0098] 2-Aminonicotinic acid (0.01 mol) was added to 25 mL of dry dichloromethane, thionyl chloride (0.05 mL) was added to reflux for 3 h, the solvent was spin-dried, chloroform was quickly added, and 5-fluoroisatin (0.01 mL) was added. mol) was added to the reaction system, and the triethylamine (0.001mol) of catalytic equivalent was added dropwise, refluxed for 2 hours, and TLC monitoring was completed. elution) to obtain the target compound 4A-2.
[0099]
[0100] Yellow solid, 62% yield. M.P.>300°C, 1H NMR (400MHz, DMSO) δ 9.09 (dd, J=4.6, 2.0Hz, 1H), 8.72 (dd, J=7.9, 2.0Hz, 1H), 8.47(dd,J=8.8,4.2Hz,1H),7.86(dd,J=7.1,2.7Hz,1H),7.76(ddd,J=11.8,6.6,2.9Hz,2H).
[0101] 13C NMR (101MHz, DMSO) δ181.95, 159.70, 158.42, 157.59, 156.75, 148.38, 142.51, 136.85, 125.34, 124.68, 124.44, 119.60, 119.23, 112.51.C14HFN3O2 [M+H6FN3O2] + , 268.0517; found, 268.0514.
Embodiment 3
[0102] Example 3 Compound 4A-3 (10-bromopyrido[2',3':4,5]pyrimido[1,2-a]indol-5(11H)-one)
[0103] 2-Aminonicotinic acid (0.01 mol) was added to 25 mL of dry dichloromethane, thionyl chloride (0.05 mL) was added to reflux for 3 h, the solvent was spin-dried, chloroform was rapidly added, and 4-bromoisatin (0.01 mL) was added. mol) was added to the reaction system, and the triethylamine (0.001mol) of catalytic equivalent was added dropwise, refluxed for 2 hours, and TLC monitoring was completed. elution) to obtain the target compound 4A-3.
[0104]
[0105] Yellow solid, 55% yield. M.P.>300°C, 1H NMR (400MHz, DMSO) δ 9.10 (dd, J=4.6, 2.0Hz, 1H), 8.73 (dd, J=7.9, 2.0Hz, 1H), 8.50(dd,J=7.8,0.9Hz,1H),7.78(ddd,6.3,4.7,3.2,2H),7.72(dd,J=8.1,0.9Hz,1H).
[0106] 13C NMR (101MHz, DMSO) δ180.39, 158.48, 157.61, 156.84, 147.79, 147.67, 138.99, 136.96, 132.01, 125.29, 121.12, 120.31, 119.48, 116.48.
[0107] C14H6BrN3O2[M+H] + ,327.9716;found,327.9715
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com