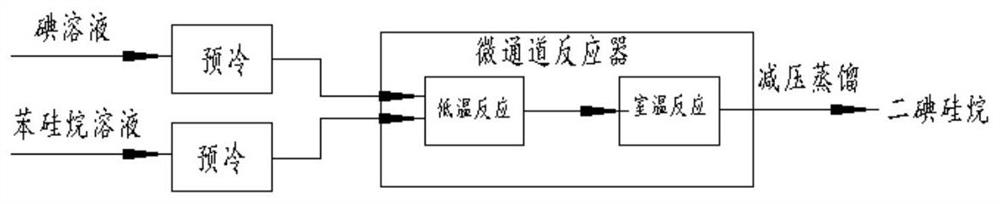
Preparation method and device of diiodosilane
A technology of diiodosilane and phenylsilane, which is applied in the field of preparation of diiodosilane, can solve problems such as difficult acquisition of raw materials, limitation of industrial production, and difficulty in product purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Chloroform is a solvent for iodine
[0047] Under nitrogen protection, a homogeneous mixed solution containing 3518.1 g of iodine and 10 L of chloroform was prepared. At the same time, a mixed solution of 1500 g of phenylsilane and 58.8 ml of ethyl acetate was pumped into the microchannel reactor respectively. 8:1, after pre-cooling at -40°C, the mixture was simultaneously passed through a low temperature microchannel at -35°C and a room temperature microchannel at +25°C, and the residence time was 200s and 300s, respectively, to obtain a mixture. The mixture was rectified to obtain 3625 g of a colorless liquid, 92.11%. The calculation method is as follows: 3625 / (1500*283.91 / 108.21)*100%=92.11%.
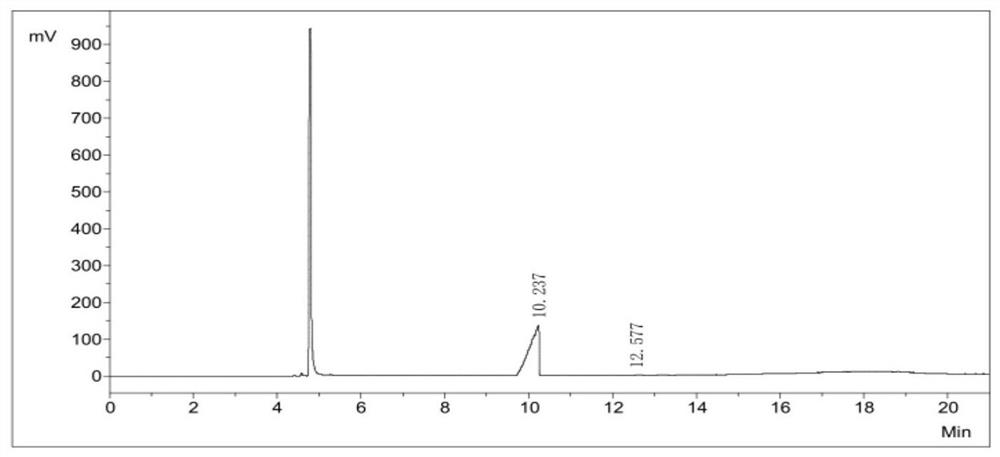
[0048] Using the GC chromatographic area normalization method, the purity of diiodosilane was 99.9+%, as shown in Table 1 and figure 2 shown.
[0049] Purity calculation of the diiodosilane of Table 1 Example 1
[0050]
Embodiment 2
[0051] Example 2: Chloroform is a solvent for iodine
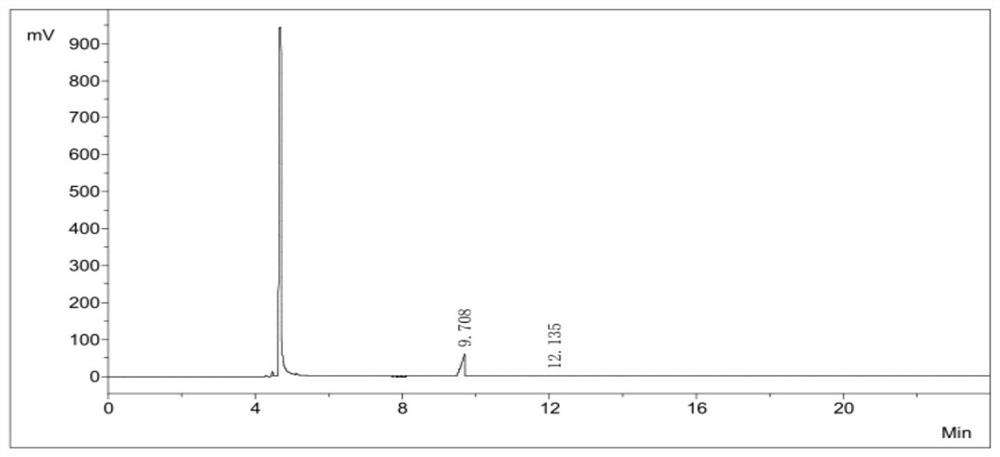
[0052] Under nitrogen protection, a homogeneous mixed solution containing 3518.1 g of iodine and 10 L of chloroform was prepared. At the same time, a mixed solution of 1500 g of phenylsilane and 58.8 ml of ethyl acetate was pumped into the microchannel reactor respectively. The volume flow ratio of the iodine solution and the phenylsilane solution was 8 : 1. After pre-cooling at -20°C, the mixture was obtained by passing through the -15°C low temperature microchannel and the room temperature microchannel at +25°C, respectively, with the residence time of 200s and 300s. The mixture was rectified to obtain 3683 g of a colorless liquid, 93.58%. Using the GC chromatographic area normalization method, the purity of diiodosilane is greater than 99.9+%, as shown in Table 2 and image 3 shown.
[0053] The purity calculation of the diiodosilane of table 2 embodiment 2
[0054]
Embodiment 3
[0055] Example 3: Chloroform is a solvent for iodine
[0056] Under nitrogen protection, a homogeneous mixed solution containing 3518.1 g of iodine and 10 L of chloroform was prepared. At the same time, a mixed solution of 1500 g of phenylsilane and 58.8 ml of ethyl acetate was pumped into the microchannel reactor respectively. The volume flow ratio of the iodine solution and the phenylsilane solution was 10 : 1. After being pre-cooled at 0°C, the mixture is obtained by passing through a low temperature microchannel at 10°C and a room temperature microchannel at +25°C, respectively, for a residence time of 100s and 300s. The mixture was rectified to obtain 3541 g of a colorless liquid, 89.97%. Using GC chromatographic area normalization method, the purity of diiodosilane is greater than 99.9+%, as shown in Table 3 and Figure 4 shown.
[0057] The purity calculation of the diiodosilane of table 3 embodiment 3
[0058]
[0059] From Example 1, Example 2, and Example 3, it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Radial width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com