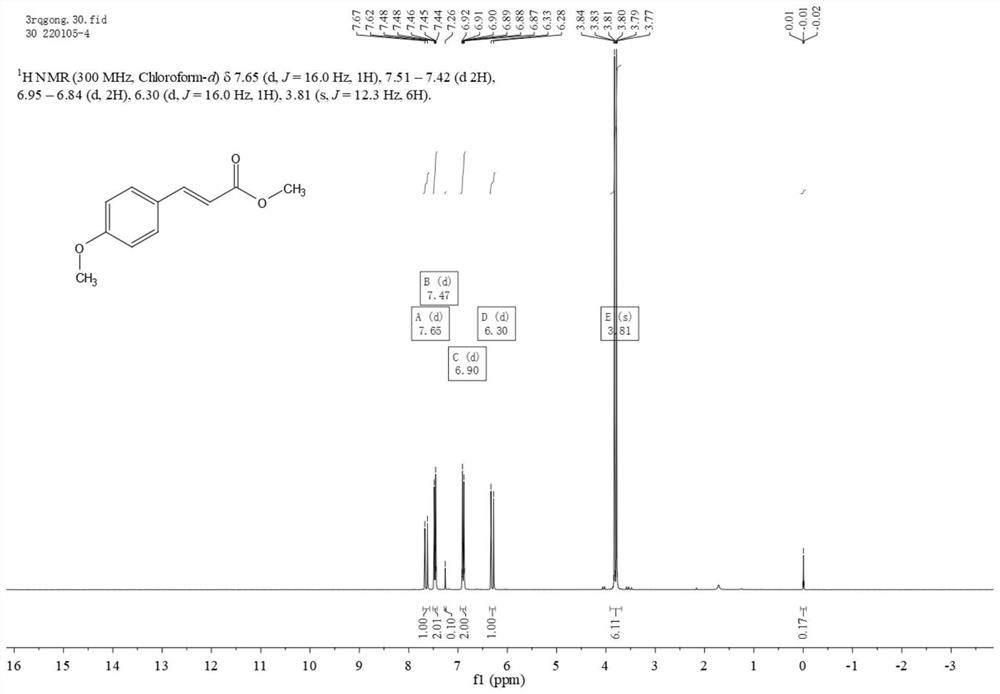
Method for synthesizing p-methoxycinnamate through concerted catalysis of small molecule free guanidine and N-methyl-2-pyrrolidone
A technology of molecular free guanidine and methoxy meat, applied in the field of antioxidant synthesis, can solve the problems of raw material loss, reduced conversion rate, low utilization rate, etc., and achieves the effects of low cost, short reaction time and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1: Small molecule guanidine preparation
[0020] First weigh 5.4g of guanidine nitrate (0.044mol) and add it to a 250ml reactor equipped with a condenser tube, stirring and drying tube, then weigh 64.8ml of absolute ethanol and add it to the guanidine nitrate-containing reactor, stir at room temperature To completely dissolve the ethanol solution of guanidine nitrate, prepare a sodium ethoxide ethanol solution with a concentration of 0.8mol / L. Finally, take 55 ml of the prepared ethanol solution of sodium ethoxide and slowly drop it into the ethanol solution of guanidine nitrate. Add dropwise while stirring, and carry out suction filtration after reaction for 2.0h. The filter cake obtained by suction filtration is white sodium chloride, and the filtrate is an ethanol solution of guanidine. The ethanol solvent is distilled off to obtain colorless crystalline small-molecule free guanidine.
[0021] Step 2: Synthesis of methyl p-methoxycinnamate
[0022] In a 250ml r...
Embodiment 2
[0025] Step 1: Small molecule guanidine preparation
[0026] First weigh 7.9g of guanidine carbonate (0.044mol) and add it to a 250ml reactor equipped with a condenser tube, stirring and drying tube, then weigh 118.5ml of absolute ethanol and add it to the guanidine carbonate-containing reactor, stir at room temperature To completely dissolve the ethanolic solution of guanidine carbonate, prepare a sodium ethoxide ethanolic solution with a concentration of 1.2 mol / L. Finally, take 48 ml of the prepared ethanolic solution of sodium ethoxide and slowly add it dropwise to the ethanolic solution of guanidine carbonate. Add dropwise while stirring, and carry out suction filtration after reaction for 1.5 hours. The filter cake obtained by suction filtration is white sodium chloride, and the filtrate is an ethanol solution of guanidine. The ethanol solvent is distilled off to obtain small molecule free guanidine in the form of colorless crystals.
[0027] Step 2: Synthesis of methyl ...
Embodiment 3
[0031] Step 1: Small molecule guanidine preparation
[0032] First weigh 9.5g of guanidine sulfate (0.044mol) and put it into a 250ml reactor equipped with a condenser tube, stirring and drying tube, then weigh 190ml of absolute ethanol and add it to the guanidine sulfate-containing reactor, stir at room temperature until Completely dissolve to obtain the ethanol solution of guanidine sulfate, and prepare a sodium ethoxide ethanol solution with a concentration of 1.5mol / L. Finally, take 44 ml of the prepared ethanol solution of sodium ethoxide and slowly add it dropwise to the ethanol solution of guanidine sulfate, drop at room temperature. After stirring, the reaction was carried out for 1 h and suction filtration was carried out. The filter cake obtained by suction filtration was white sodium chloride, and the filtrate was an ethanol solution of guanidine. The ethanol solvent was distilled off to obtain small molecule free guanidine in the form of colorless crystals.
[0033...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com