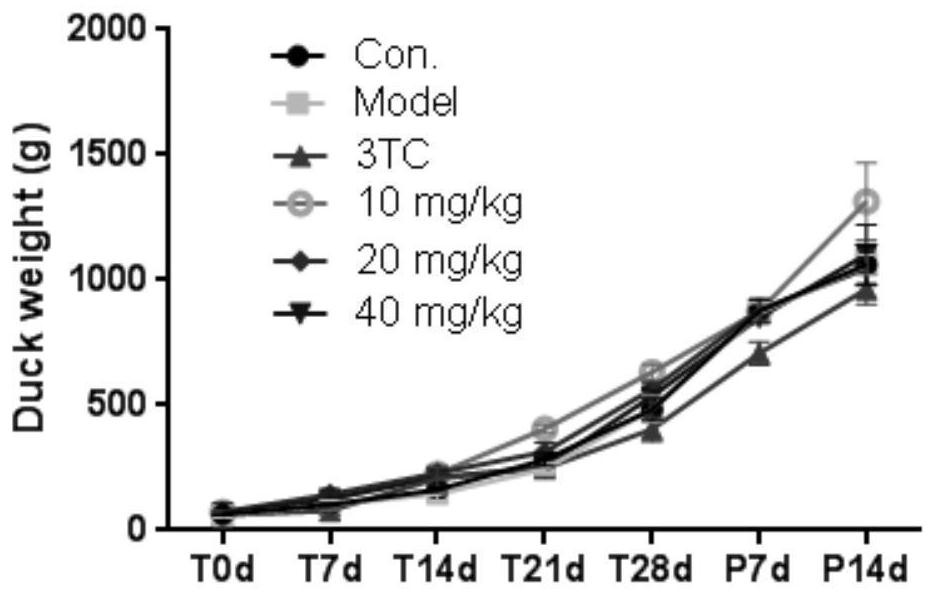
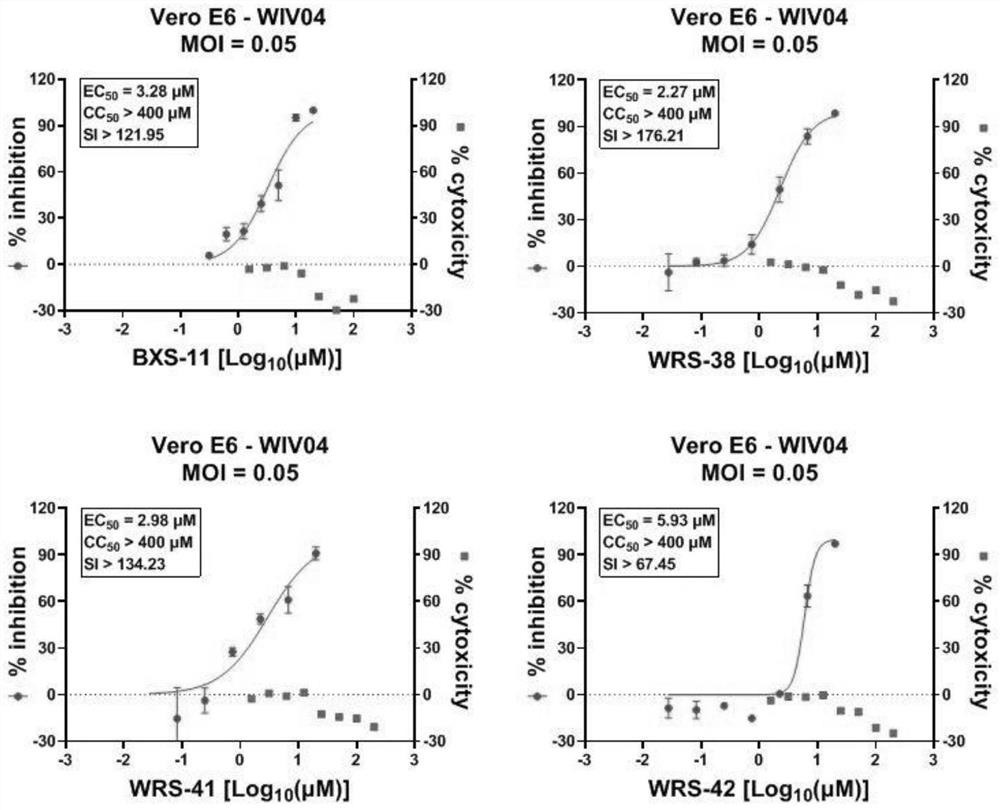
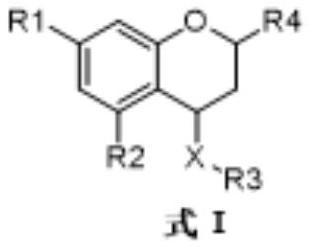
Benzodihydropyran compound with anti-hepatitis B virus and anti-coronavirus effect
A technology of chroman and dihydropyran, which is applied in antiviral agents, organic chemistry, medical preparations containing active ingredients, etc., to achieve the effect of easy raw materials, good inhibitory effect, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the compound shown in the formula I of the present invention includes but is not limited to the preparation method of the compound shown in the formula II and the compound shown in the formula III. The preparation method of the compound shown in the formula II: the compound shown in the formula IV is The raw material is reacted with copper bromide using ethyl acetate as a solvent under reflux conditions to obtain the brominated product shown in formula V. Then, the brominated product shown in formula V and triphenylphosphorus were dissolved in ethyl acetate, and reacted at room temperature to obtain a white solid; sodium hydroxide solution (2N) was added to the dichloromethane solution in which the white solid was dissolved, and adjusted pH to 12. After stirring at room temperature for 30 min, the compound represented by formula VI can be obtained. The compound represented by the formula VI and the aldehyde are dissolved in dichloromethane, an...
Embodiment 1
[0072] Example 1: Preparation of Intermediate I-1
[0073]
[0074] 2,4-Dihydroxyacetophenone (15.21 g, 0.1 mol) was dissolved in 1 L of acetone and potassium carbonate (55.2 g, 0.4 mol) was added. Dimethyl sulfate (0.11 mol) was added in an ice bath, the dropwise addition was completed, the temperature was returned to room temperature, and the mixture was heated to reflux in an oil bath for 5 hours. After the reaction was completed, 500 mL of ice water was added, and the mixture was extracted three times with ethyl acetate. The organic phases were combined and washed three times with saturated NaCl, then dried over anhydrous magnesium sulfate. After being concentrated by distillation under reduced pressure, intermediate I-1 was obtained after purification by silica gel chromatography with a yield of 90%.
[0075] 1 H-NMR (400MHz, CDCl 3 ),δ12.75(s,1H),7.63(d,J=8.7Hz,1H), 6.44(dd,J=8.7,2.5Hz,1H),6.42(d,J=2.4Hz,1H),3.84 (s,3H),2.56 (s,3H). 13 C-NMR (100MHz, CDCl 3)δ20...
Embodiment 2
[0076] Example 2: Preparation of Intermediate I-2
[0077]
[0078] Intermediate I-1 (16.62 g, 0.1 mol) was dissolved in ethyl acetate (1500 mL), copper bromide was added at room temperature, and heated under reflux for 12 h. After cooling to room temperature, the mixture was filtered and neutralized with saturated aqueous sodium bicarbonate solution. After three extractions with ethyl acetate, the organic phases were combined, washed three times with saturated NaCl, dried over anhydrous magnesium sulfate, and concentrated by distillation under reduced pressure to obtain the crude product. Then after purification by silica gel chromatography and recrystallization, intermediate I-2 was obtained in 90% yield.
[0079] 1 H NMR (400MHz, CDCl 3 )δ12.22(s, 1H), 7.65(d, J=8.9Hz, 1H), 6.48(dd, J=8.9, 2.5Hz, 1H), 6.45(d, J=2.5Hz, 1H), 4.36( s,2H),3.86(s,3H). 13 C-NMR (100MHz, CDCl 3 )δ195.3, 167.1, 166.4, 132.1, 111.3, 108.5, 101.3, 55.8, 29.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com