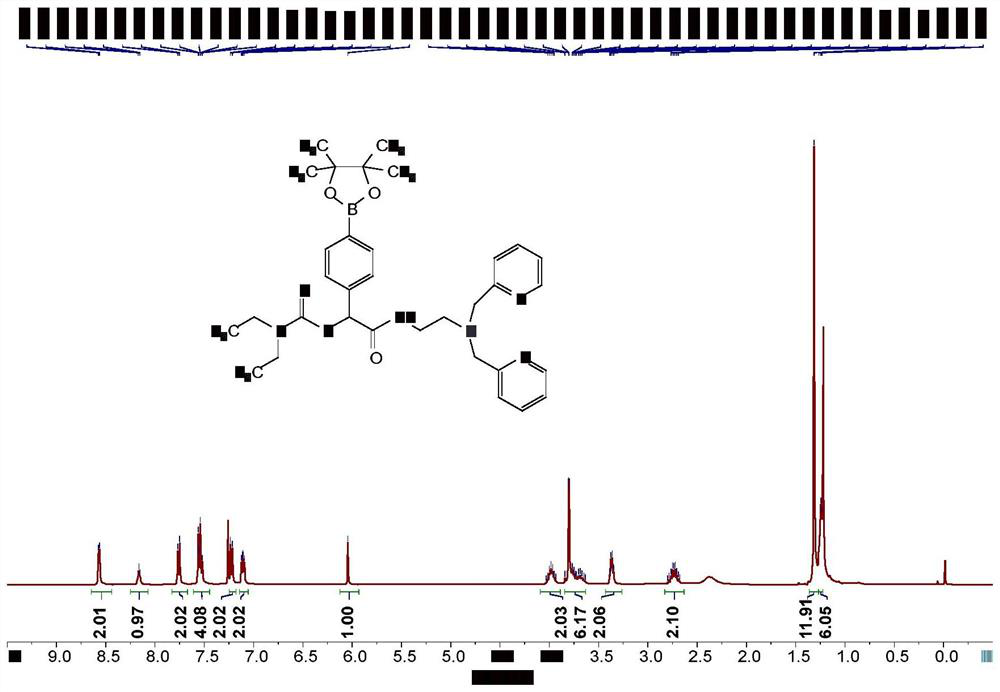
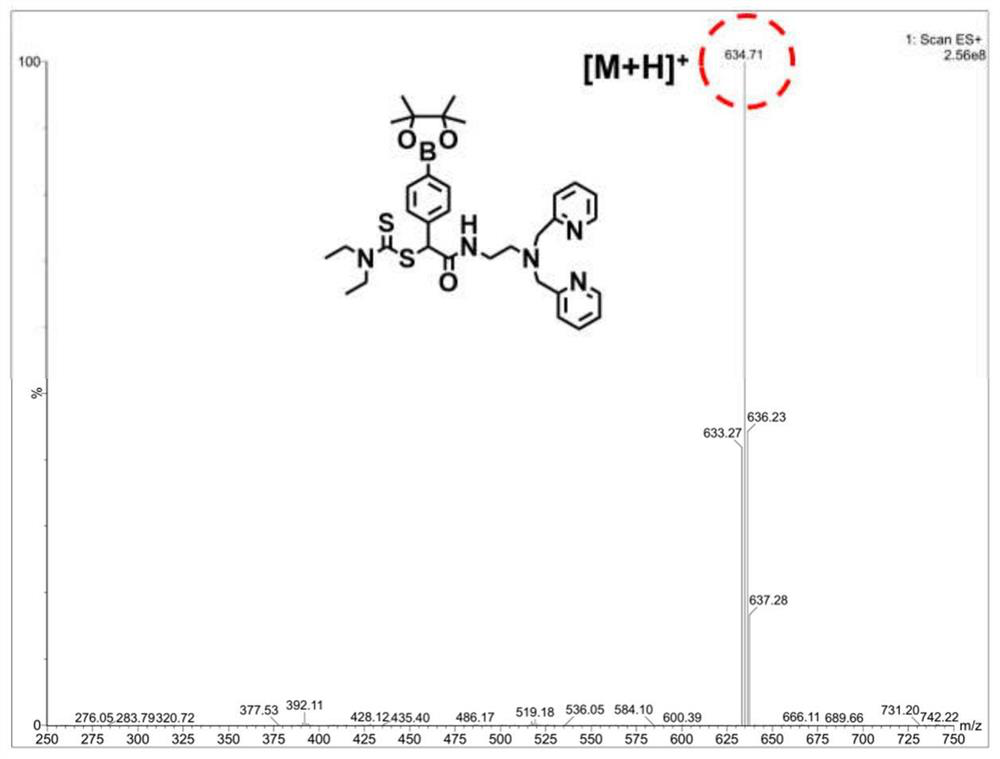
Prodrug compound of dithiocarbamic acid metal complex as well as preparation method and application of prodrug compound
A technology of dithiocarbamic acid and metal complexes, applied in the field of pharmacy, can solve the problem that prodrugs are limited to certain specific stimuli or disease environments, achieve good clinical practicability, good druggability, and overcome single design types Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Preparation of ROS-sensitive copper diethyldithiocarbamate complex prodrug compound DPBD-Cu prepare
[0078] (1) The preparation process of DPBD compound is as follows:
[0079]
[0080] 1) Preparation of intermediate compound 1: mono-Boc-ethylenediamine (4.00g, 24.97mmol, 1eq), 2-chloromethylpyridine hydrochloride (9.01g, 54.93mmol, 2.2eq) and anhydrous sodium sulfate (13.23g) g, 124.84 mmol, 5 eq) was dissolved in 200 mL of methanol, and heated to reflux for 72 h under nitrogen protection. After the reaction was completed, the solvent was evaporated, redissolved in dichloromethane, and suction filtered. The filtrate was concentrated and purified by silica gel column chromatography (dichloromethane / methanol=20:1).
[0081] 2) DPA-NH 2 Preparation: Intermediate compound 1 (6.00g, 17.52mmol, 1eq) was dissolved in 150mL of dichloromethane, placed in an ice bath, trifluoroacetic acid (26ml, 20eq) was slowly added dropwise under the ice bath, and the re...
Embodiment 2
[0089] Example 2: Preparation of ROS-sensitive copper diethyldithiocarbamate prodrug compound DPPBD-Cu prepare
[0090] (1) The preparation process of DPPBD compound is as follows:
[0091]
[0092] 1) Preparation of intermediate compound 7: 2,6-bis(hydroxymethyl)-p-cresol (10g, 59.46mmol, 1eq) and imidazole (8.91g, 130.80mmol, 2.2eq) were dissolved in 40mL of anhydrous DMF, Place in ice bath. Tert-butyldimethylsilyl chloride was dissolved in 40 mL of anhydrous DMF, dropped into the above reaction solution under an ice bath, and stirred at room temperature for 2 h. The reaction solution was diluted with 200 mL of ethyl acetate, and washed three times with water (100 mL×3). The organic phase was collected, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated and purified by silica gel column chromatography (petroleum ether:ethyl acetate=50:1).
[0093] 2) Preparation of intermediate compound 8: Intermediate compound 7 (5.11 g, 12...
Embodiment 3
[0098] Example 3: Preparation of negative control DPD-Cu
[0099] (1) The preparation process of DPD compound is as follows:
[0100]
[0101] 1) Preparation of intermediate compound 11: α-Bromophenylacetic acid (0.50g, 2.33mmol, 1eq) was dissolved in 5mL of anhydrous acetonitrile, sodium diethyldithiocarbamate (0.42g, 2.44mmol, 1.05eq) was dissolved in In 5 mL of anhydrous acetonitrile, drop it into the above solution, and stir the reaction at room temperature until a white precipitate forms. After completion of the reaction, the reaction solution was filtered, concentrated, redissolved in 25 mL of double distilled water, adjusted to pH value of about 5 with 1M HCl, extracted with dichloromethane (50 mL×3), and collected all the dichloromethane layers. Dry with anhydrous sodium sulfate, suction filtration, and concentrate the filtrate to obtain.
[0102] 2) Preparation of DPD: intermediate compound 11 (0.425 g, 1.50 mmol, 1 eq) and DPA-NH 2 (0.436g, 1.80mmol, 1.2eq) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com