High-stereoselectivity R transketolase mutant as well as coding gene and application thereof
A stereoselective, gene-encoding technology, applied in the field of enzyme engineering, can solve problems such as low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Site-directed and saturation mutagenesis and screening
[0037] Site-directed mutagenesis and saturation mutagenesis of natural transketolase derived from prokaryotic Escherichia coli and screening for R-transketolase with high activity and high stereoselectivity. The nucleotide sequences of the primers used are shown in Table 1 below. In Table 1, N represents any base of A, T, C and G, K represents the base of T or G, and the NNK degenerate codon can encode 20 random amino acids . M stands for A or C base, and MNN is complementary to NNK.
[0038] Table 1 Primer Nucleotide Sequences
[0039]
[0040] (1) Using the recombinant plasmid pET28a-TK as a template, the nucleotide sequence of which is shown in SEQ ID No. 3, and using D469T-F and D469T-R as primers, Phanta Max polymerase (purchased from Novozan) was used to carry out PCR amplification (95°C for 5 min; 95°C for 30s, 65°C for 30s, 72°C for 7.5min, 34 cycles; 72°C for 10min); PCR products were dig...
Embodiment 2
[0045] Example 2: Expression and purification of transketolase mutants
[0046] The expression and purification method of the mutant EcTK1_YYH: pick a single colony of E. coli BL21 (DE3) containing the recombinant plasmid on LB solid medium and inoculate it into 40ml LB liquid medium (containing 50μg / ml kanamycin antibiotic) , 37 ℃, 220rpm culture overnight. Transfer 7.5ml of bacterial culture solution to a 2L shake flask containing 500ml of liquid LB medium, inoculate two flasks, culture at 37°C, 220rpm until OD600 reaches 0.6-0.8, add 0.4mM IPTG for induction, and induce culture at 30°C, 200rpm 5h. 1 L of fermentation broth was collected, cells were collected by centrifugation at 5000 rpm for 20 min, and the collected cells were resuspended in 30 mL of nickel column binding buffer and placed in an ice-water mixture. Ultrasonic fragmentation conditions: work for 5s, pause for 10s, and a total of 30min. The crushed mixture was centrifuged at 12,000 rpm for 1 h, and the supe...
Embodiment 3
[0047] Example 3: Investigating the stereoselectivity of EcTK1 mutants catalyzing p-methylsulfonylbenzaldehyde to obtain products
[0048] The highly stereoselective R-transketolase mutant EcTK1 catalyzes the detection process of p-methylsulfonylbenzaldehyde to obtain highly stereoselective products, and the involved chemical reactions include transketone reaction and transamination reaction.
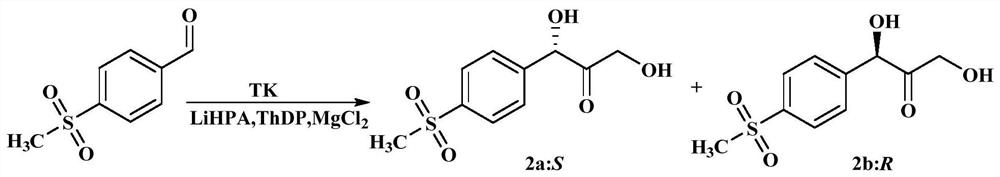
[0049] like figure 1 As shown, the principle of the transketone reaction is to use p-methylsulfonylbenzaldehyde as the substrate, the substrate is represented by "1", the transketolase mutant is the catalyst, the catalyst is represented by "TK", LiHPA, MgCl 2 In the presence of ThDP, S-dihydroxyketone and R-dihydroxyketone products are generated, S-dihydroxyketone is represented by "2a:S", and R-dihydroxyketone product is represented by "2b:R".
[0050] like figure 2 As shown, the principle of the transamination reaction is to instantaneously convert the dihydroxyketone, the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com