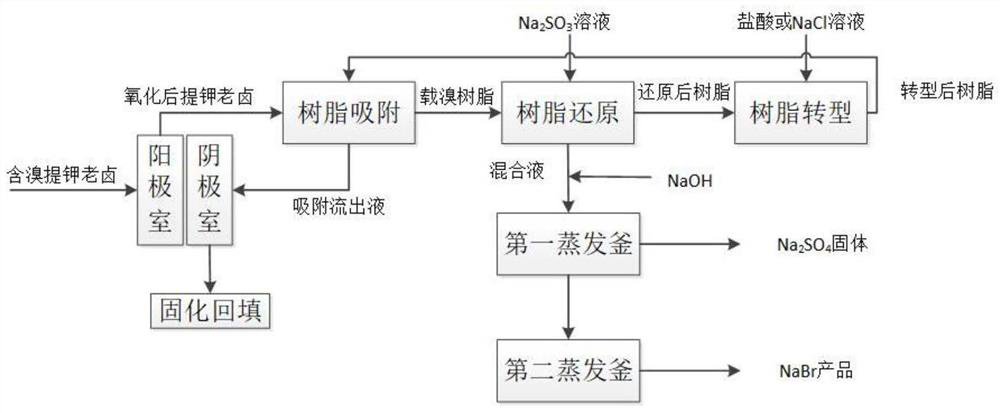
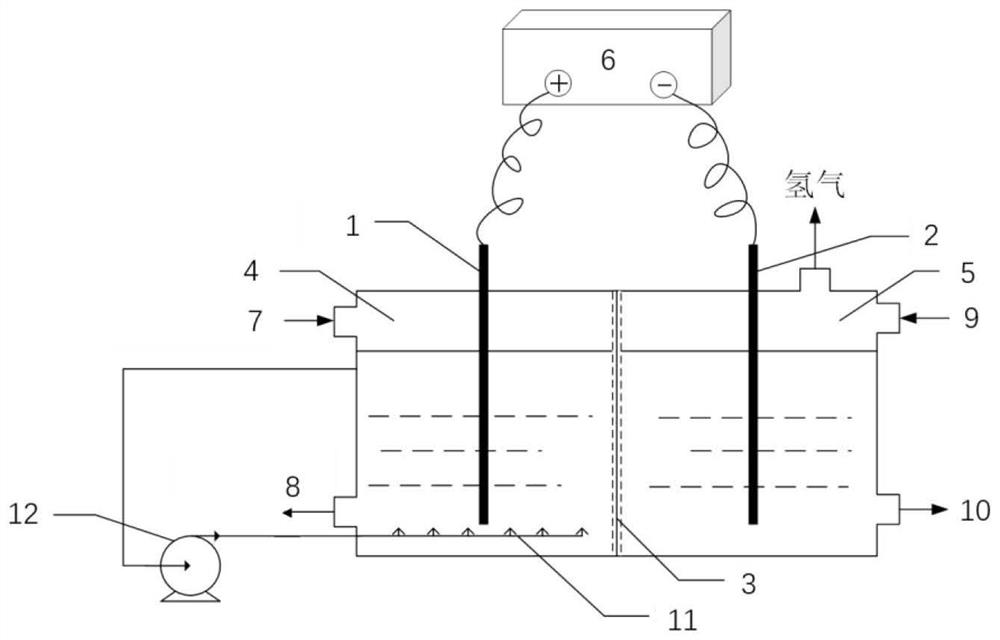
Method for extracting bromine from potassium extraction old brine and producing sodium bromide
A technology of sodium bromide and old halogen, applied in the direction of alkali metal bromide, chemical industry, improvement of process efficiency, etc., to avoid storage and transportation safety problems and environmental pollution risks, avoid pH increase, and the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Adopt the above-mentioned method provided by the invention to extract bromine and prepare sodium bromide from the old brine containing bromine and potassium, and its brine composition and concentration are:
[0050]
[0051] The specific experimental process is as follows:
[0052] (1) the anode chamber of ion-exchange membrane two-chamber electrolysis device is passed into the old halogen containing bromine and potassium, and the anode material in the described anode chamber is a graphite electrode; The resin adsorption effluent is passed into the cathode chamber, and the negative electrode in the described cathode chamber is passed into the cathode chamber. The material is platinum metal electrode; at 40A / m 2 In the constant current mode, a direct current is passed through the two-chamber electrolysis device of the ion exchange membrane, and the bromine ion undergoes an electrolysis reaction at the anode to generate elemental bromine;
[0053] (2) pass the pretrea...
Embodiment 2
[0059] Adopt the above-mentioned method provided by the invention to extract bromine and prepare sodium bromide from the old brine containing bromine and potassium, and its brine composition and concentration are:
[0060]
[0061] The specific experimental process is as follows:
[0062] (1) the anode chamber of ion-exchange membrane two-chamber electrolysis device is passed into the old halogen containing bromine and potassium, and the anode material in the described anode chamber is a graphite electrode; The resin adsorption effluent is passed into the cathode chamber, and the negative electrode in the described cathode chamber is passed into the cathode chamber. The material is platinum metal electrode; at 50A / m 2 In the constant current mode, a direct current is passed through the two-chamber electrolysis device of the ion exchange membrane, and the bromine ion undergoes an electrolysis reaction at the anode to generate elemental bromine;
[0063] (2) pass the pretrea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com