Synthesis method of tetraalkyl tetrazaporphyrin metal coordination compound
A technology of tetraalkylporphyrazine and alkylporphyrazine, which is applied in the field of synthesis of metal complexes, can solve the problems of low yield and poor solubility, and achieve high yield, high-efficiency synthesis method, The effect of easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] As above-mentioned specific synthetic steps:
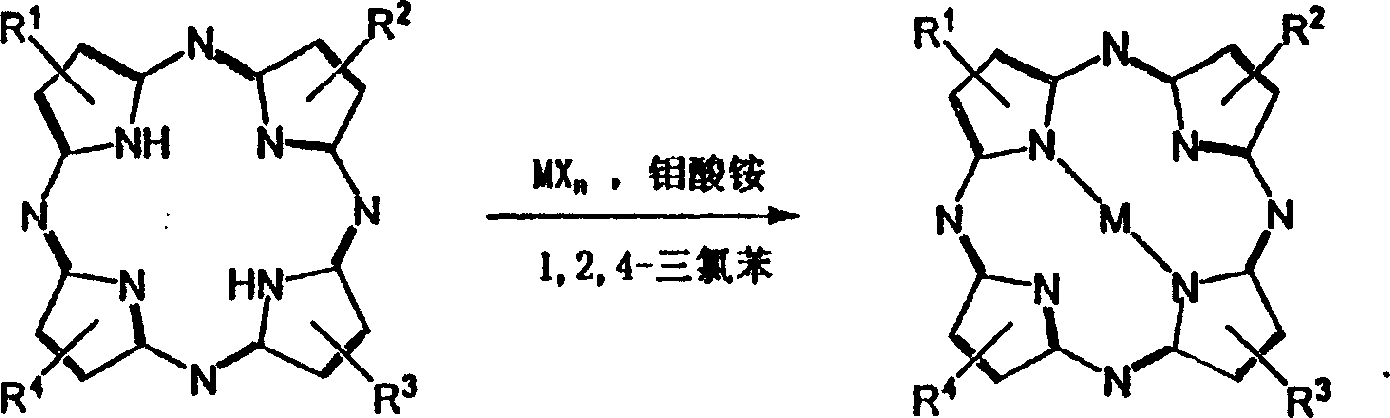
[0019] (1) The raw material that takes by weight percentage as follows: Tetraalkyl porphyrazine tetra-tert-butyl porphyrazine 1.5%, 1,2,4-trichlorobenzene 96%, anhydrous cuprous chloride 2%, ammonium molybdate 0.5%, the sum of the total weight percentages is 100%;
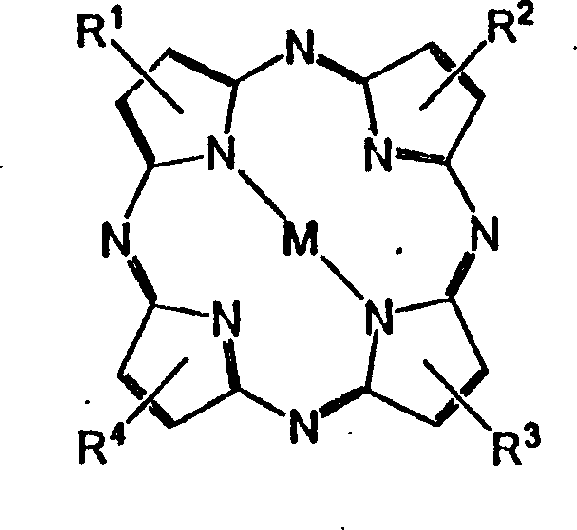
[0020] (2) Dissolve tetra-tert-butyl porphyrazine, anhydrous cuprous chloride, and ammonium molybdate in 1,2,4-trichlorobenzene, boil for 4 hours, and monitor the reaction with electronic absorption spectroscopy Proceeding situation, reaction formula is as above.
[0021] (3) After the reaction, the solvent was distilled off under reduced pressure, and a silica gel column was used for chromatographic separation to obtain a deep purple tetra-tert-butylporphyrazine copper complex.
[0022] Yield: 90.8%, melting point>200°C.
[0023] The characteristic absorption peaks of the infrared spectrum are: 2955, 2922, 2863, 1247, 1200, 771, 843, 1010 (the strongest abso...
Embodiment 2
[0031] (1) The raw material that takes by weight percentage as follows: tetraalkyl porphyrazine selects tetra-tert-butyl tetraazaporphyrin 1.5%, 1,2,4-trichlorobenzene 93%, anhydrous lead acetate 5%, Ammonium molybdate 0.5%, the sum of the total weight percentages is 100%;
[0032] (2) tetra-tert-butyl porphyrazine, anhydrous lead acetate, ammonium molybdate were dissolved in 1,2,4-trichlorobenzene, under boiling conditions for 10 hours, monitored the progress of the reaction with electronic absorption spectroscopy, The reaction formula is as above.
[0033] (3) After the reaction, the solvent was distilled off under reduced pressure, and the silica gel column was used for chromatographic separation to obtain a dark green tetra-tert-butylporphyrazine lead complex.
[0034] Yield: 94.3%, melting point > 200°C.
[0035] The characteristic absorption peaks of infrared spectrum are: 2953, 2915, 2863, 1246, 1203, 771, 843, 991 (the strongest absorption), 1070 (double peak), 1358,...
Embodiment 3
[0043] (1) Weigh the following raw materials in percent by weight: tetraalkyl porphyrazine tetra-tert-butyl tetraazaporphyrin 1.5%, 1,2,4-trichlorobenzene 94%, anhydrous nickel chloride 4 %, ammonium molybdate 0.5%, the sum of the total weight percentages is 100%;
[0044] (2) Dissolve tetra-tert-butyl porphyrazine, anhydrous nickel chloride, and ammonium molybdate in 1,2,4-trichlorobenzene, boil for 12 hours, and monitor the progress of the reaction with electronic absorption spectroscopy In this case, the reaction formula is as above.
[0045] (3) After the reaction, the solvent was distilled off under reduced pressure, and the silica gel column was used for chromatographic separation to obtain a dark purple tetra-tert-butylporphyrazine nickel complex.
[0046] Yield: 93.4%, melting point > 200°C.
[0047] The characteristic absorption peaks of infrared spectrum are: 2954, 2922, 2863, 1252, 1203, 772, 843, 1013 (the strongest absorption), 1079 (double peak), 1358, 1387, 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com