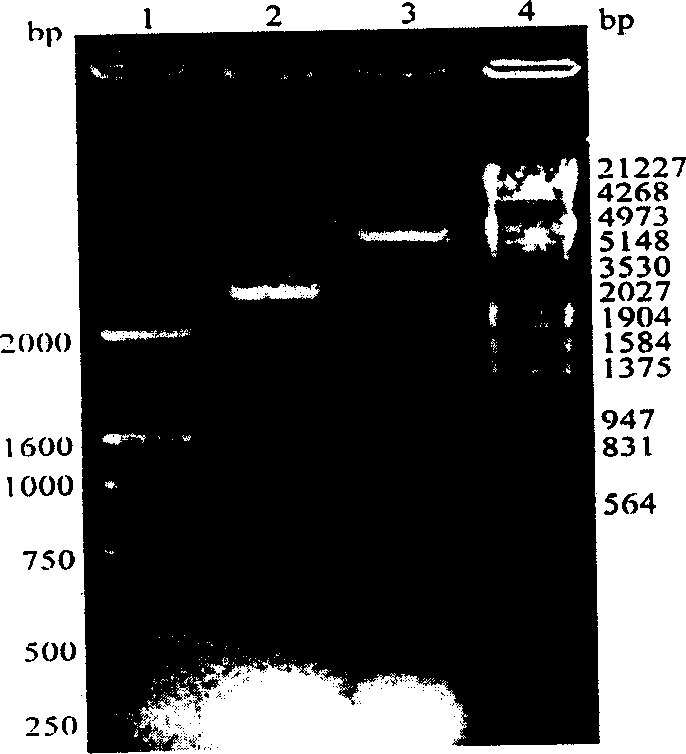
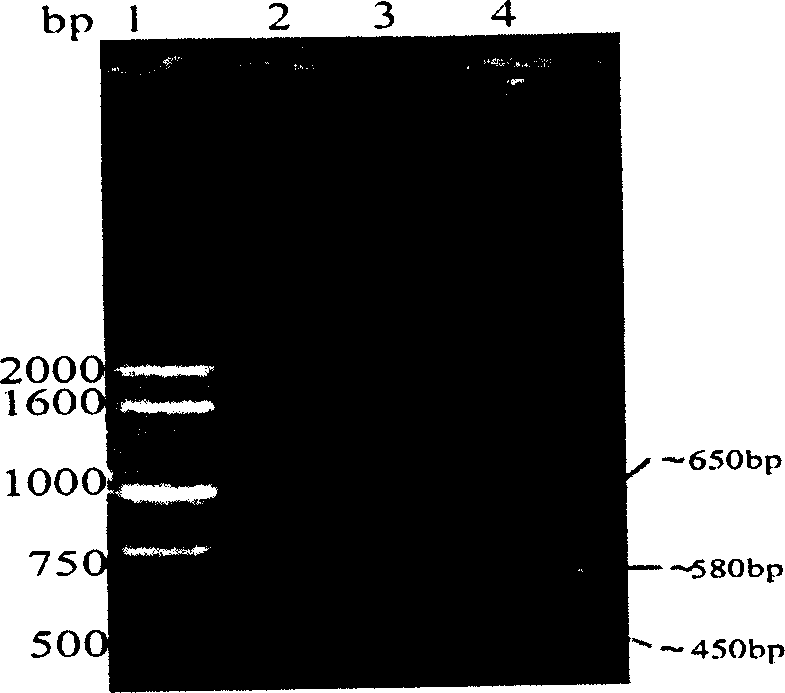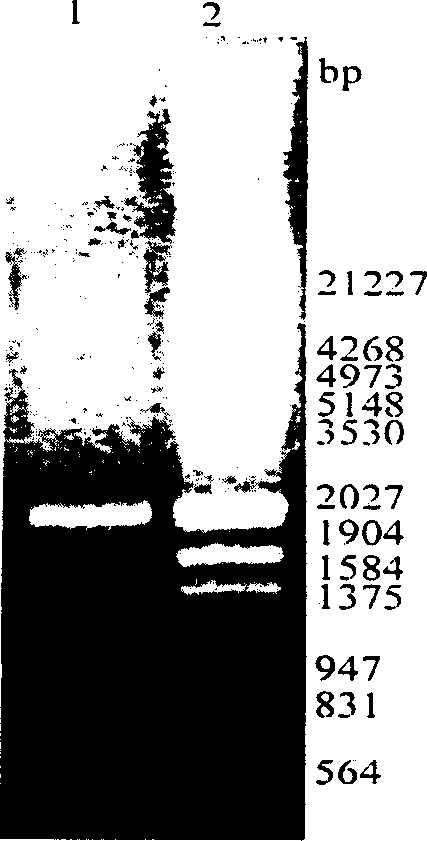Method for producing blood vessel diseases gene medicine-blood vessel endothelium growth gene-2 naked DNA by microorganism cloning vehicle
A carrier and gene technology, applied in the field of recombinant DNA and its construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Mouse-derived VEGF-2 gene and its protein identification in microbial expression system
[0034] A pair of specific primers were designed according to the known mVEGF-2 nucleotide sequence (GenBank: S38100, gi: 249860), and BamH I and EcoR I sites were respectively introduced at the 5' ends of the pair of primers.
[0035] Bam HI
[0036] VP-1: 5'-GCC TCC GGA TCC ATG AAC TTT CTG-3'
[0037] VP-2: 5'- GAA TTC ACC GCC TCG GCT TGT C-3'
[0038] Eco RI
[0039] The embryos were taken out from the mice that had been sacrificed and conceived for 2 weeks, and the total RNA extraction kit (GIBCO company product-TRIZOL) was used to extract the embryos. Reagent, Cat.No.15596) to extract total RNA. Using total embryonic RNA as a template, Oligo(dT) 15 As a primer, the first strand of cDNA was synthesized under the action of AMV Reverase (AMV-RT). Then, the reverse transcription product was diluted 50 times as a template, and the ...
Embodiment 2
[0046] Example 2. Cloning of human VEGF promoter and construction of transient expression vector for green fluorescent protein (GFP) gene and its functional identification
[0047] The upstream sequence of the human VEGF gene (hVEGF) has been reported (GenBank: AF095785, gi: 4154290), which is 3401bp, and it is difficult to clone such a large fragment. According to the general characteristics of eukaryotic promoters, the main elements of the promoter are usually about 1kb upstream of the transcription initiation site. According to the research of Hideo et al. (Hideo K, et al. Blood, 2000, 95: 189-197), we further analyzed that the fragment -1041~-1 (sorted from the transcription start site) includes the basic elements of the promoter. element. However, in the study of Hideo et al. (Hideo K, et al. Blood, 2000, 95: 189-197), the 5' untranslated region (5'-UTR) (referring to the + 1~+1038 fragments), and now it is increasingly recognized that 5'-UTR plays a very important role...
Embodiment 3
[0063] Example 3. Construction of hVEGF promoter and VEGF-2 mammalian expression vector and ischemic model animal experiment
[0064] 1. Construction of hVEGF promoter and mVEGF-2 mammalian expression vector
[0065] pEGSH (product of STRATAGENE Company of the United States, agent of MERCK Company of Germany, Cat. No. #217461) was selected as the expression vector for inserting hVEGF promoter and mVEGF-2. The reason for choosing this vector is mainly due to three considerations: ① The plasmid has a ColE1 replication origin and an Amp selection marker. It is a relaxed plasmid and can exist in multiple copies in microorganisms, which is conducive to the efficient production of microorganisms hVEGF promoter and mVEGF-2 naked DNA; the presence of selectable markers can prevent the loss of recombinant plasmids in the host bacteria, which is conducive to maintaining the stability of engineering bacteria; ②Multiple cloning sites (MCS) are available to facilitate the insertion of fore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com