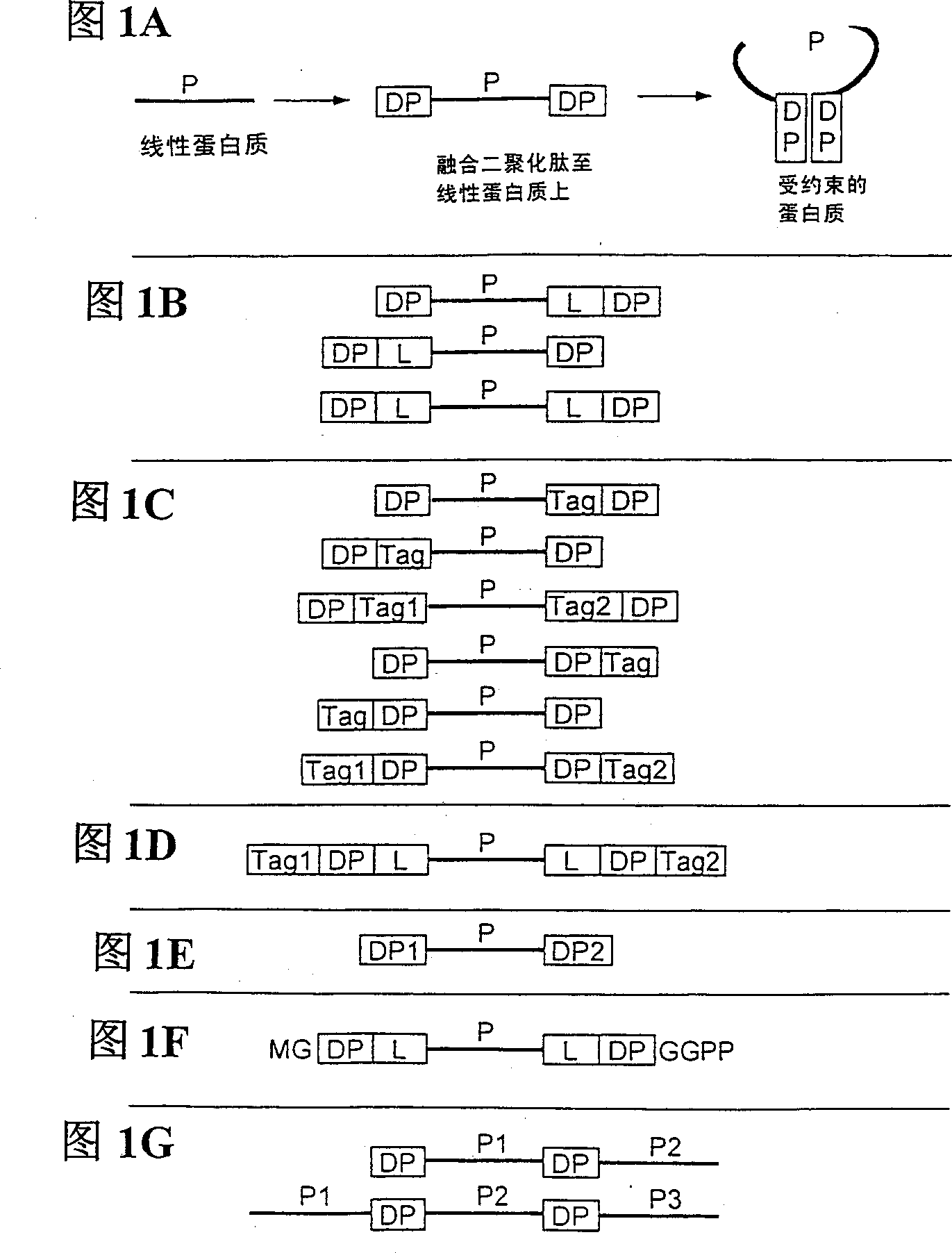
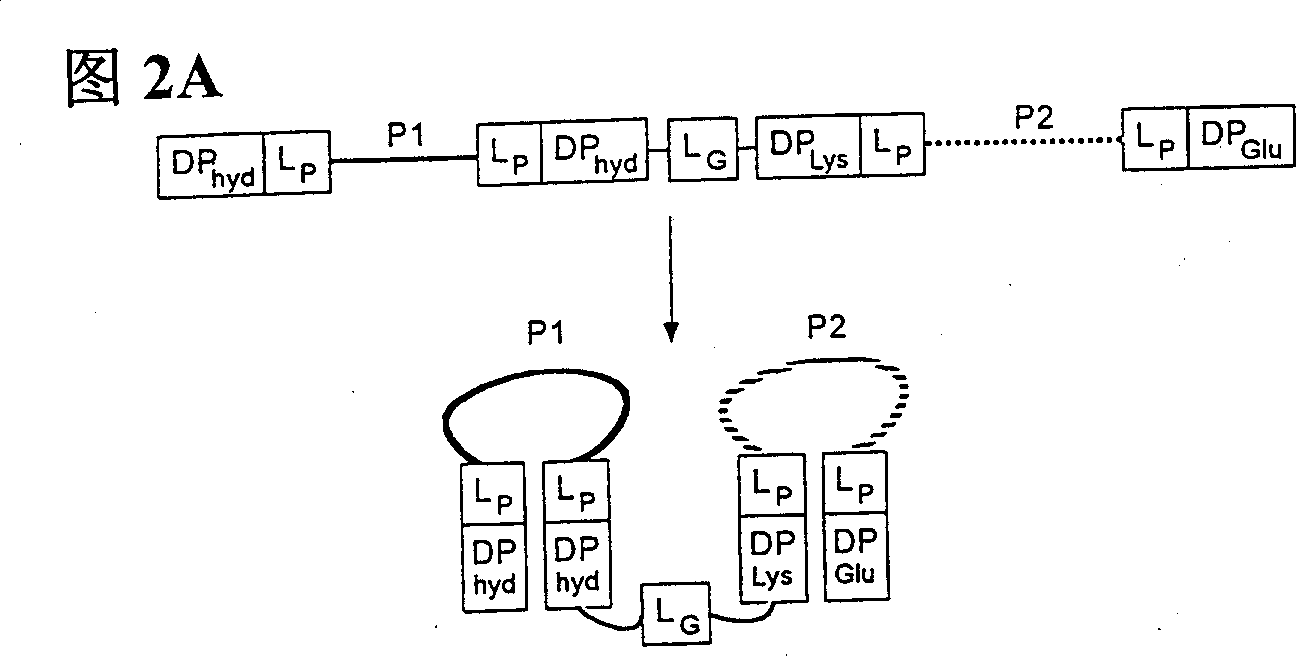
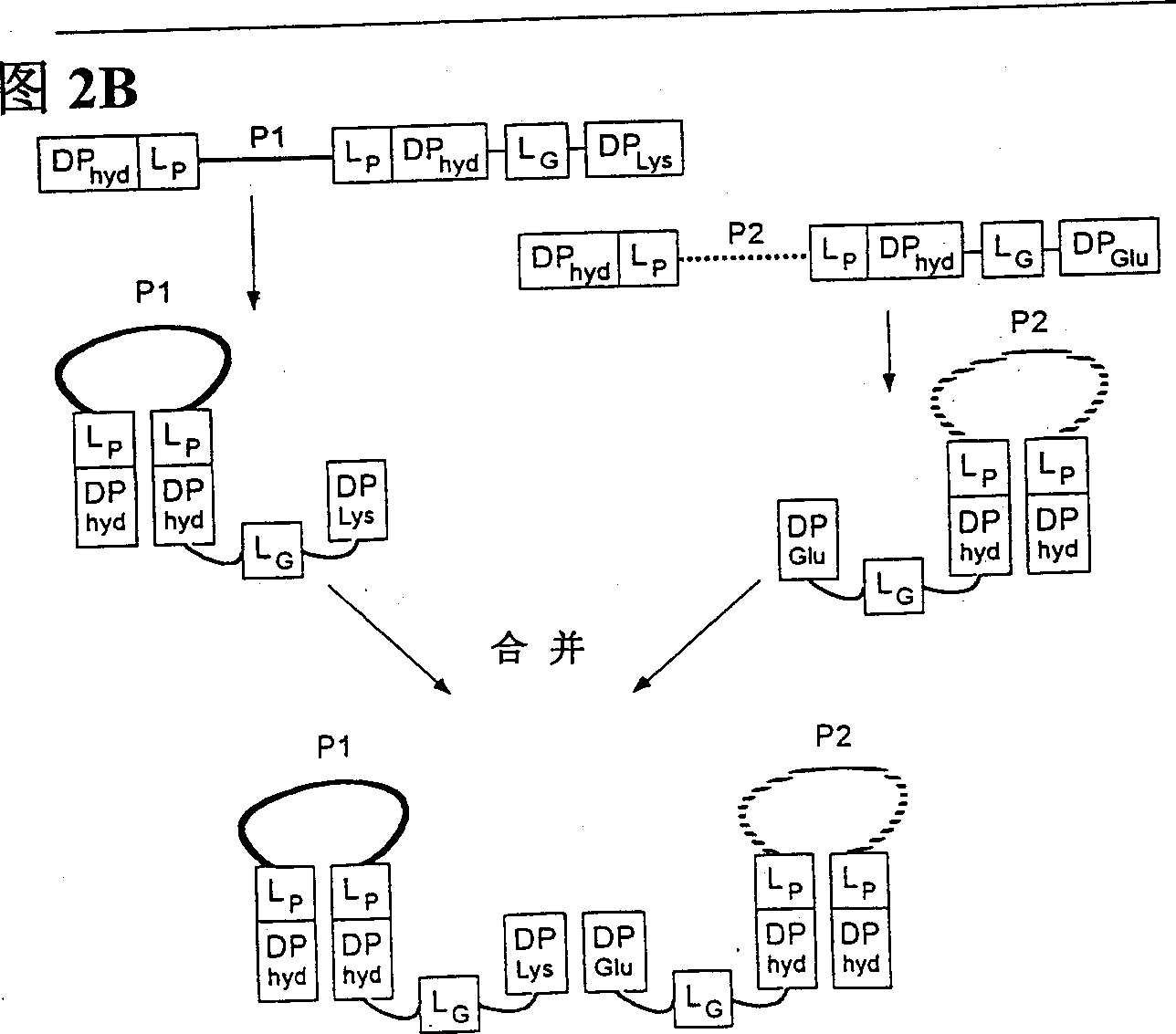
Peptides causing formation of compact structure
A member, said technology, applied in the field of peptides causing the formation of compact structures, able to solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] Novel peptides that form observable dimers under harsh conditions
[0170] By perfusing 3 x 10 -4 M, the EFLIVKS-amide solution of pH 6.4 is entered in the Finnigan LCQ ion trapping mass spectrometer of electrospray source, and this peptide appears to self-associate to form dimer ( Figure 3A ), after a temperature of 210 °C in the inlet capillary and harsh electrospray conditions, the molecular weight was detected in the gas phase to be exactly twice the molecular weight of the monomer, so it is expected to dimerize at significantly lower concentrations in aqueous solution. This peptide also forms a dimer (also detectable by mass spectrometry) when eluted from a C18 reverse phase column at about 25% acetonitrile at about pH 2.5 ( Figure 3B ). When both are continuously infused into the ion trap mass spectrometer through the electrospray interface, the comparison Figure 3A and detect the peptide SKVILFE (it is in 10 -13 M in aqueous solution to form dimers (Bodenm...
Embodiment 2
[0173] Example 2 When adding 18mer detection peptide N- and C-terminal
[0174] EFLIVKS forms compact proteolysis-resistant structures
[0175] When fused to the N- and C-termini of the 18mer detection polypeptide, the peptide EFLIVKS can form a compact structure of this polypeptide (also referred to herein as peptide 1). The 18mer polypeptide sequence is VGTIVTMEYRIDRRTRSFV from barley c2-chymotrypsin inhibitor [Leatherbarrow and Salacinski, Biochemistry 30:10717-21 (1991)]. This peptide analog, with cysteines substituted for valines at both the N- and C-termini, is thought to fold into a compact structure similar to the loop in chymotrypsin-2. Such a compact structure should be a poor substrate for proteases such as elastase, and has indeed been suggested as an inhibitor of both variants of elastase, chymotrypsin and subtilisin. This disulfide cyclized analog has been synthesized and tested by us and is actually a poor protease substrate, but still ...
Embodiment 3
[0177] Detection of low-energy conformers of peptide 1
[0178] To examine the structural properties of the compact structure of peptide 1, low energy conformers were obtained using high temperature molecular dynamics simulated annealing methods similar to those published in Nilges et al., Protein Engineering 2:27-38 (1988), as performed in Discover95. Structure saved every 2psec (from 900K, different trajectories with duration 400-1400psec), cooled to 300K with 5ps time, and distance-dependent dielectric constant (ε=80 at 80 angstrom interval to ε=1 at 1 angstrom interval linear variation between ) with 200 steps of the steepest descent algorithm for minimization, and then use as many steps as necessary with the gradient algorithm to give the largest amount of derivatives less than 0.001 kcal / A. Collect and compare the resulting low-energy structures from orbitals starting from the following structures:
[0179] a) Peptide 1, the 18mer polypeptide derived fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com