Cefetamet pivoxil hydrochloride dispersion dispersion tablets and preparation method
A technology of ceftametoxo pivoxil hydrochloride and dispersible tablets, which is applied in the field of pharmaceutical compositions and its preparation, can solve the problems of complex process, slow onset of action, and slow dissolution, and achieve high bioavailability, fast absorption, and high production efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 g%
[0031] Active ingredient Ceftazidime pivoxil hydrochloride 125 26.8
[0032] Filler microcrystalline cellulose 320 68.7
[0033] Binder Hypromellose 1.0 0.21
[0034] Disintegrant Crospovidone 10 2.1
[0035]
[0036] Lubricant Magnesium stearate 3 0.6
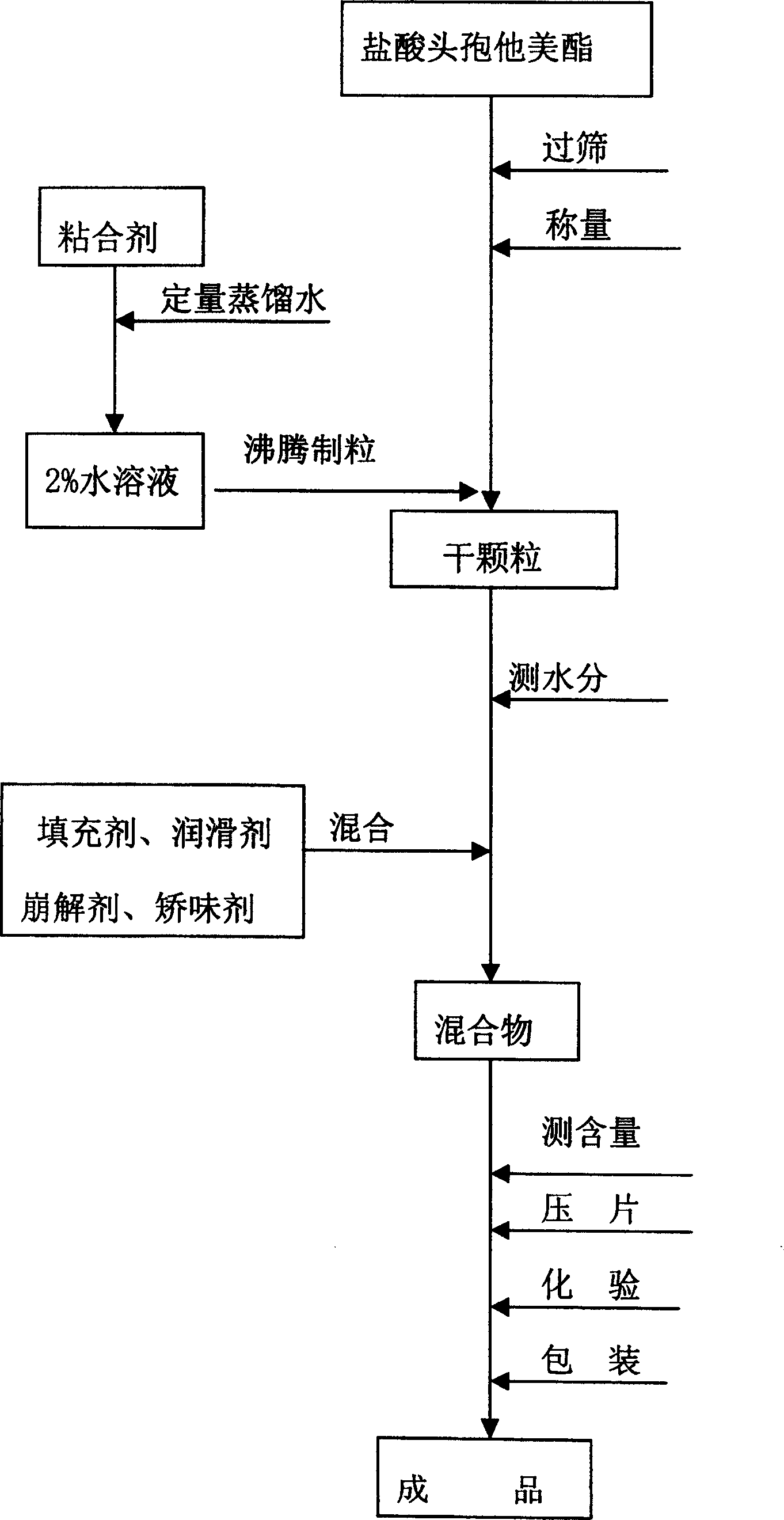
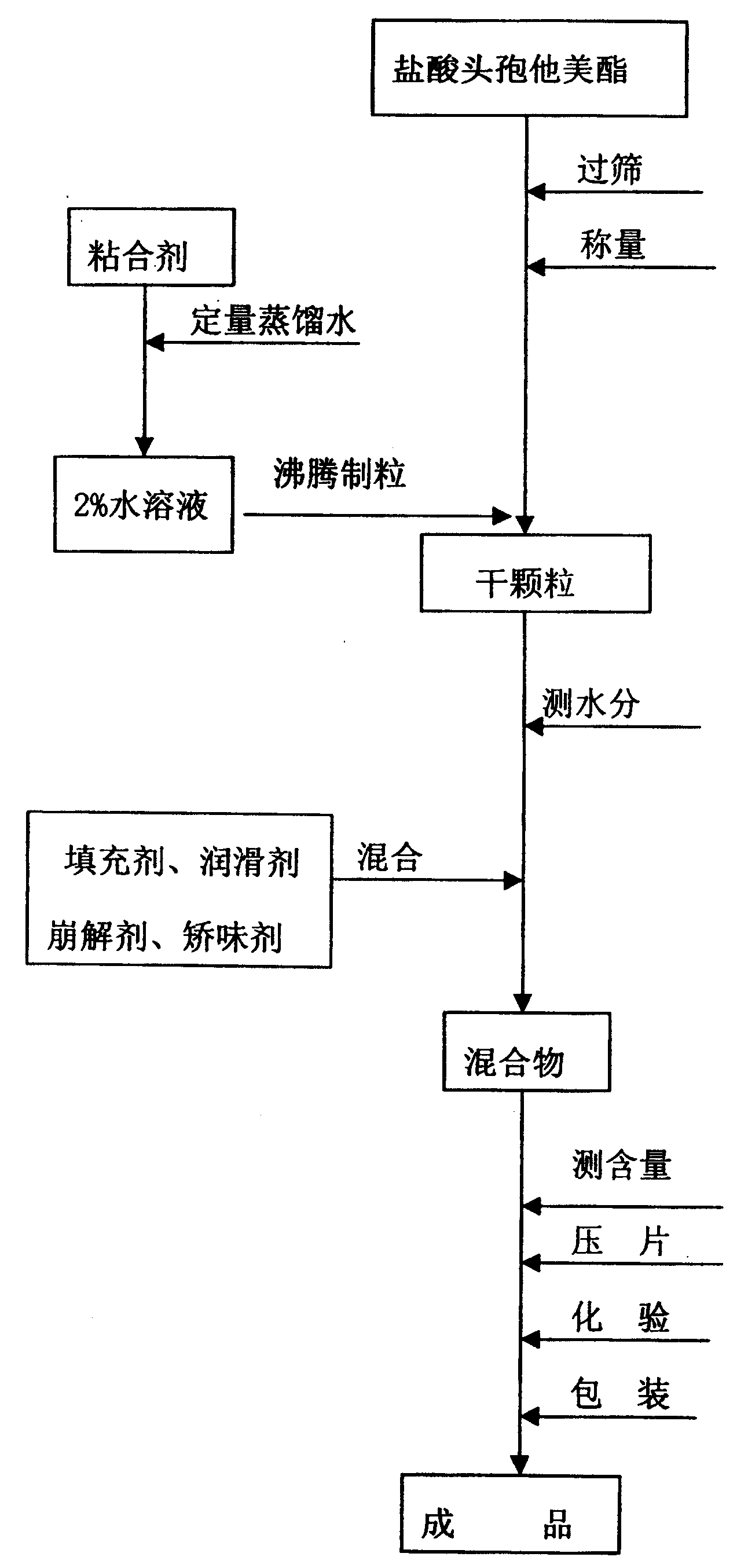
[0037] Preparation method: 1. Preparation of adhesive (1): Measure 100 ml of water, add 1.0 g of hypromellose, and stir to dissolve it.
[0038] 2. Preparation of granules: After passing through a 120-mesh sieve of ceftazidime pivoxil hydrochloride, weigh 125g and spray it into the barrel of a boiling granulator to inject the adhesive (1) for boiling granulation. The dry granules are granulated through a 30-mesh sieve .
[0039] 3. Tablet preparation: add 320g microcrystalline cellulose, 10g crospovidone, 5g bitterness masking agent, 2g mint essence, and 3g magnesium stearate, mix well, make 1000 tablets, 466mg / tablet; contain hydrochloric acid Ceftazidime pivoxil 125mg / tablet.
example 2
[0040] Example 2: g %
[0041] Active ingredient Ceftazidime pivoxil hydrochloride 200 34.1
[0042]Filler Pregelatinized starch 345 58.9
[0043] Binder Hypromellose 2.0 0.34
[0044] Disintegrant Croscarmellose Sodium 25 4.3
[0045]
[0046] Lubricant Magnesium stearate 3 0.51
[0047] 1. Preparation of adhesive (2): Measure 100ml of water, add 2.0g of hypromellose, stir to dissolve
[0048] 2. Preparation of granules: After passing ceftazidime pivoxil hydrochloride through a 120-mesh sieve, weigh 200 g and spray it into the barrel of a boiling granulator to inject the binder (2) for boiling granulation. The dry granules are granulated through a 30-mesh sieve .
[0049] 3. Preparation of tablets: Add 345g precrosslinked starch, 25g croscarmellose sodium, 9g bitterness masking agent, 2g mint flavor, and 3g magnesium stearate, mix well, make 1000 tablets, 586mg / Tablets; containing cefetamet pivoxil hydrochloride 200mg / tablet.
example 3
[0050] Example 3: g%
[0051] Active ingredient Ceftazidime pivoxil hydrochloride 250 30.5
[0052]
[0053] Binder Povidone 3.0 0.37
[0054]
[0055]
[0056] Lubricant Magnesium stearate 8 0.98
[0057] Preparation of the adhesive (3): Measure 100 ml of water, add 3.0 g of povidone, and stir to dissolve it.
[0058] 1. Preparation of granules: After sieving ceutaxel hydrochloride, weigh 250g, put it in the barrel of the boiling granulator, spray into the binder (3), carry out boiling granulation, and the dry granules are granulated through a 30-mesh sieve.
[0059] 2. Preparation of tablet: add 400g microcrystalline cellulose, 120g calcium hydrogen phosphate, 15g crospovidone, 10g sodium starch glycolate, 10g aspartame, 4g mint essence, and 8g magnesium stearate, mix Uniform, made into 1000 tablets, tablet weight 820mg, containing cefetamet pivoxil hydrochloride 250mg / tablet.
[0060] Detection: the in vitro dispersion, dissolution and stability tests of the ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com