Tumor necrosis factor-gamma
A nucleic acid molecule and sequence technology, applied in antitumor drugs, fungi, DNA/RNA fragments, etc., can solve problems such as unclear regulation of blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
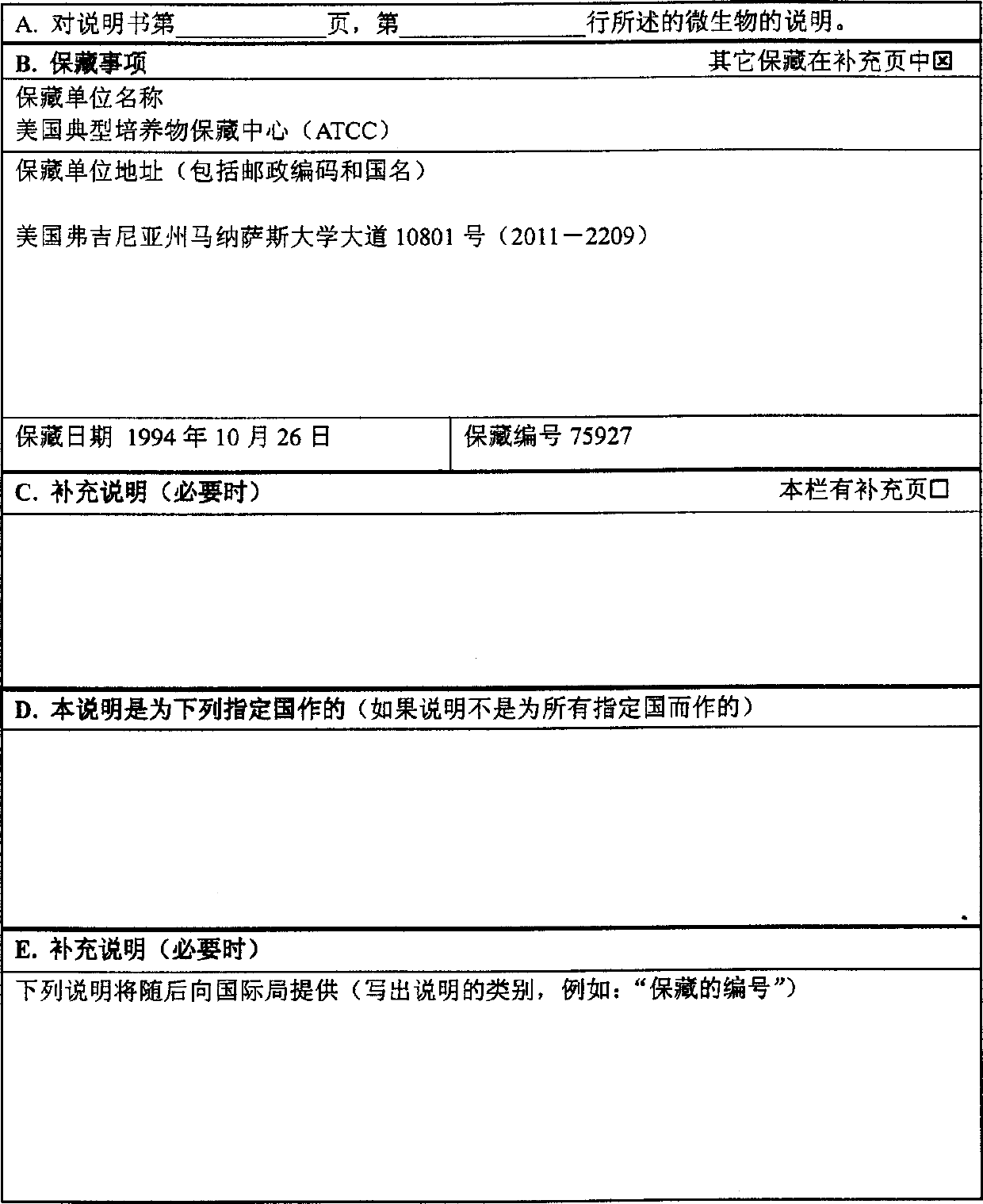
[0391] Embodiment 1, bacterial expression and purification of TNF-γ
[0392] The DNA sequence encoding the full-length TNF-γ ORF, ATCC Accession No. 75927 was first amplified with PCR oligonucleotide primers corresponding to the 5' and 3' sequences of the TNF-γ protein. Additional nucleotides corresponding to TNF-γ were added to the 5' and 3' sequences, respectively. The 5' oligonucleotide primer is shown as SEQ ID NO: 3, the sequence is 5'-GCGCGGATCCACCATGAGACGCTTTTTAAGCAAAGTC-3', which contains a -BamHI restriction enzyme site followed by the TNF-γ code starting from the initial methionine codon The first 24 nucleotides of the sequence. The 3' sequence is 5'-CGCGTCTAGACTATAGTAAGAAGGCTCCAAAGAAGG-3' (SEQ ID NO: 14), which contains an XbaI site and a sequence of 22 nucleotides complementary to TNF-γ. This restriction enzyme site corresponds to the restriction enzyme site in the bacterial expression vector pQE-9 (Qiagen), which was then digested with BamHI and XbaI. The ampli...
Embodiment 2
[0394] Embodiment 2: Clone and express TNF-γ with the baculovirus expression system
[0395] The DNA sequence (ATCC NO.75927) encoding the full-length TNF-γ protein is amplified with PCR oligonucleotide primers corresponding to the 5' and 3' sequences of the gene, and the 5' primer sequence is 5'-GCGCGGATCCACCATGAGACGCTTTTTAAGCAAAGTC-3' (SEQ ID NO: 15), which contains a -BamHI restriction enzyme site (in bold) followed by 24 nucleotides of the TNF-γ gene. The 3' primer sequence is 5'-CGCGTCTAGACTATAGTAAGAAGGCTCCAAAGAAGG-3' (SEQ ID NO: 16), which contains a restriction endonuclease XbaI site and 22 nucleotides complementary to the 3' untranslated sequence of the TNF-γ gene, amplified Sequences were isolated from 1% agarose gels using a commercially available kit ("Geneclean", BIO 101 Inc., La Jolla, CA). This fragment was then digested with endonucleases BamHI and XbaI and purified on a 1% agarose gel. This fragment is called F2.
[0396] Utilize the baculovirus expression s...
Embodiment 3
[0404] Embodiment 3. Expression of recombinant TNF-γ in COS cells
[0405]The expression of plasmid TNF-γ-HA was derived from a vector pcDNA I / Amp (Invitrogen) containing: 1) SV40 origin of replication, 2) ampicillin resistance gene, 3) E coli origin of replication, 4) CMV promoter sub, followed by a linker region, a SV40 intron and a polyadenylation site. A DNA fragment encoding the entire TNF-γ precursor and a hemagglutinin antigen (HA) tag fused to the 3' end of its framework was cloned into the polylinker region of the vector. Expression of the recombinant protein is thus under the control of the CMV promoter. The HA tag corresponds to an epitope derived from the influenza hemagglutinin protein (I. Wilson, H. Niman, R. Heighten, A Cherenson, M. Connolly, and R. Lerner, 1984, Cell 37, 767). Fusion of the HA tag to the target protein allows easy detection of the recombinant protein with antibodies recognizing HA epitopes.
[0406] The mechanism of plasmid construction is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com