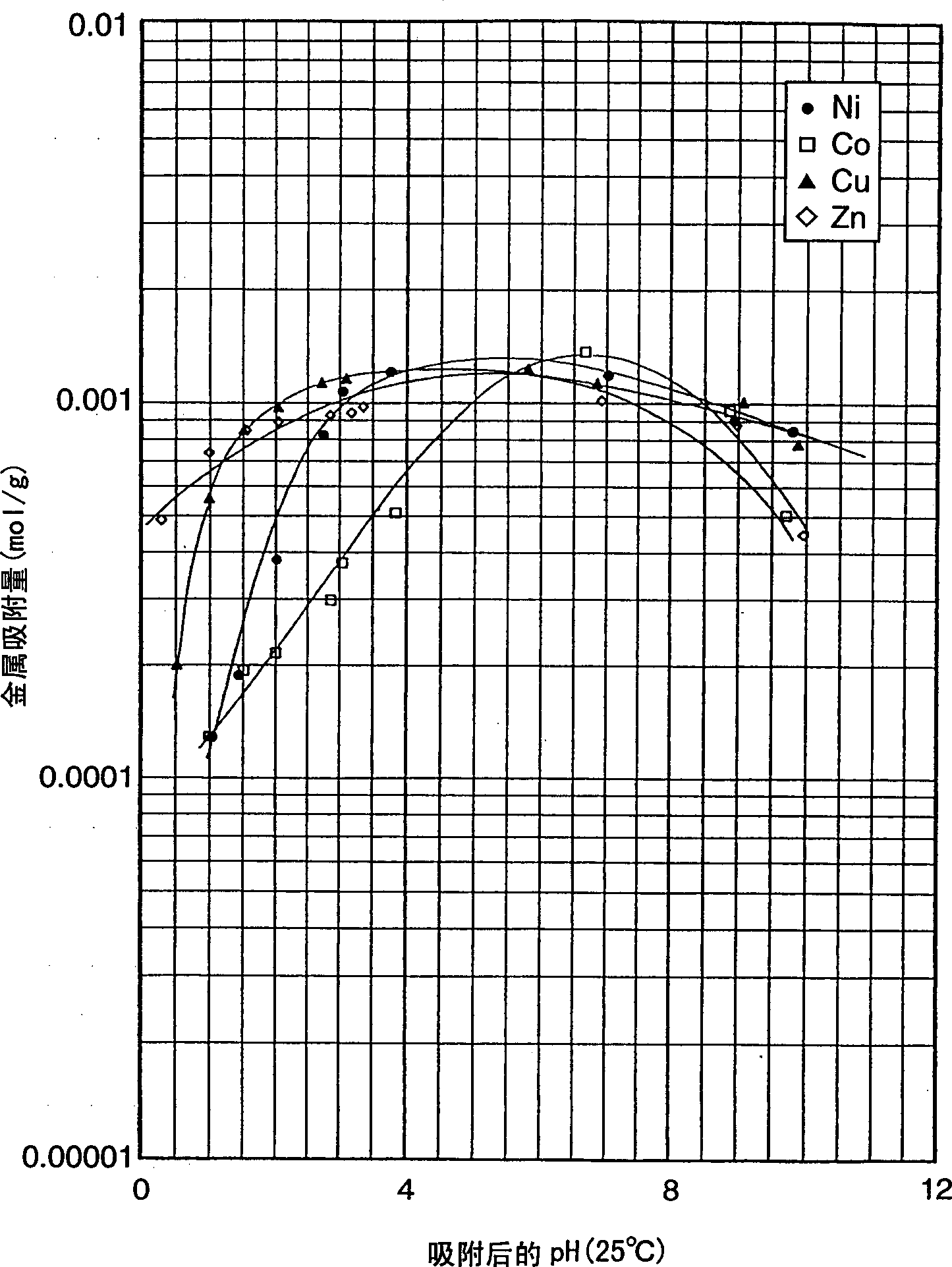
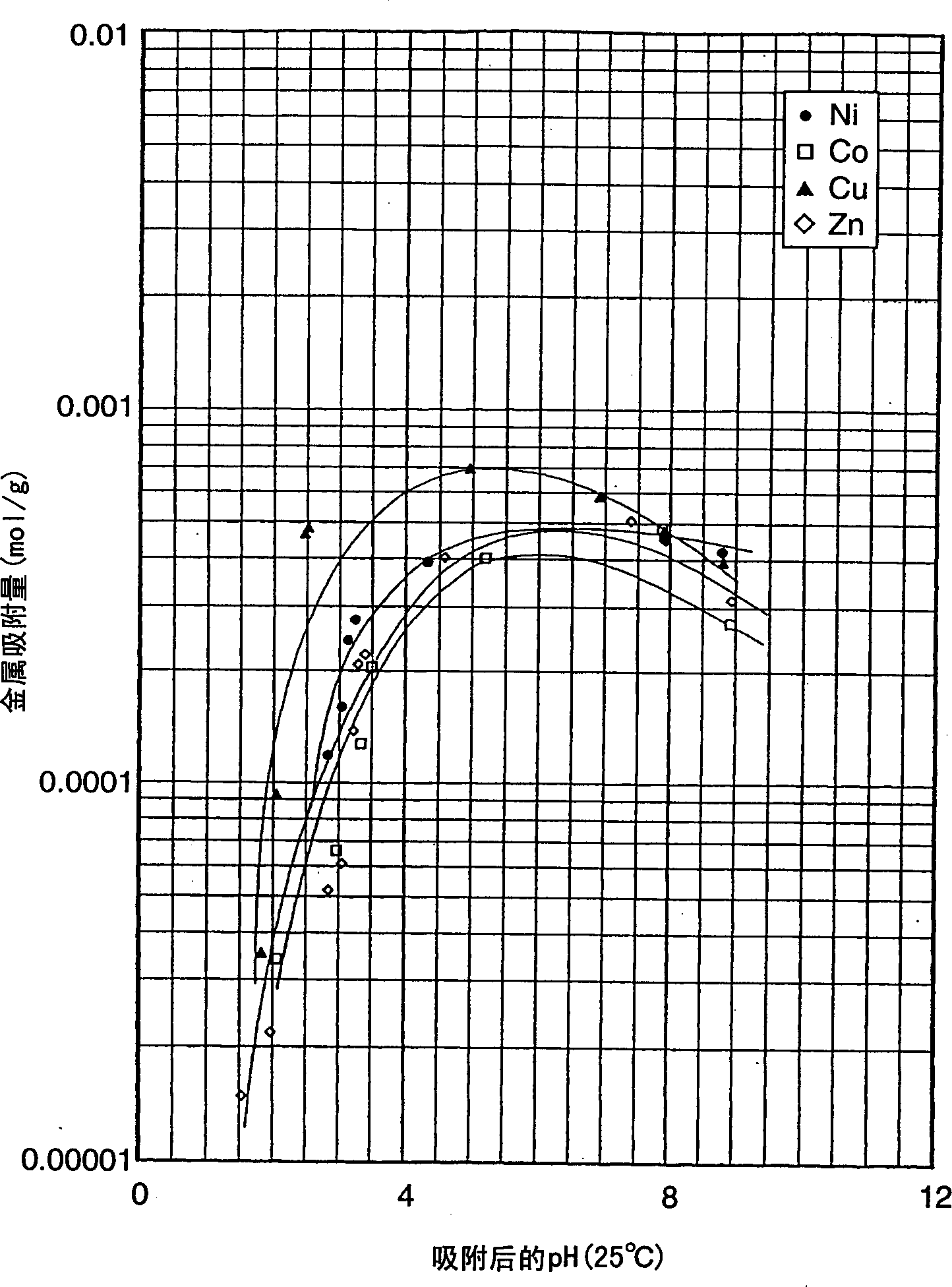
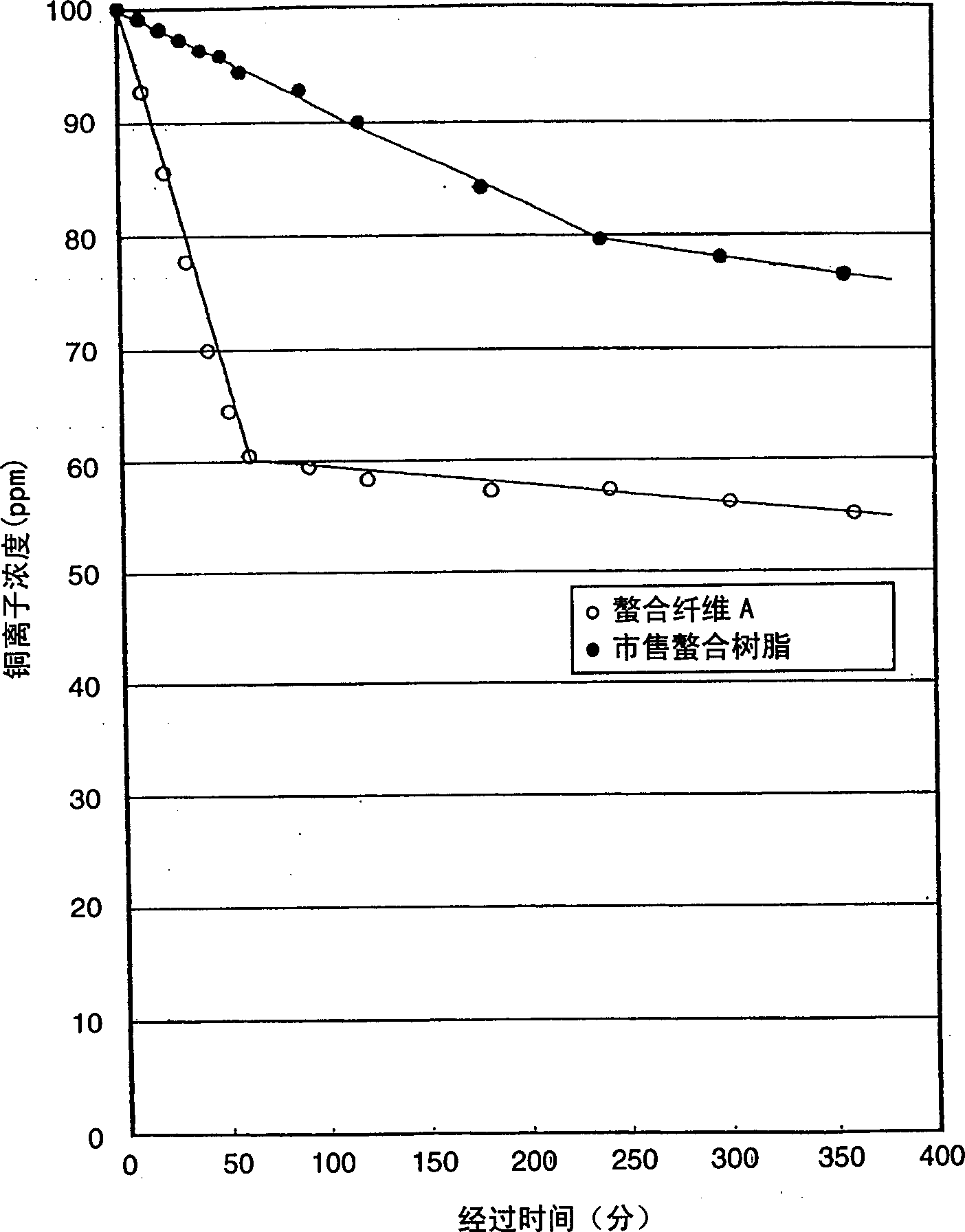
Fiber capable of forming metal chelate process for producing the same, method of trapping metal ion with the fiber, and method chelate fiber
A technology of metal chelates and metal ions, which is applied in the ion exchange of chelates, plant fibers, ion exchanges, etc., can solve the problems of dioxins and other problems, and achieve the effect of being easy to discard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 0.2 g of ferrous ammonium sulfate hexahydrate was dissolved in 800 ml of distilled water, and 20 g of cotton cloth (unbleached cotton fabric) was immersed in the solution at 20° C. for 15 minutes, and then centrifuged to remove the liquid. On the other hand, 80 ml of distilled water, 6 g of glycidyl methacrylate, 0.3 g of a nonionic surfactant (manufactured by NOF Corporation, trade name "Nonionic OT-221"), 0.7 g of 31% hydrogen peroxide, and sulfur dioxide 0.25 g of urea was emulsified and dissolved to make a solution, and the cotton cloth from which the liquid was removed by centrifugation was soaked in the solution, and treated at 60° C. for 2 hours. Next, the treated cotton cloth was washed with distilled water, centrifuged to remove the liquid, and then dried at 60° C. for 16 hours to obtain 24.8 g of cotton cloth grafted with glycidyl methacrylate.
[0075] Next, 200 g of iminodiacetic acid was added to 800 g of distilled water, the pH was adjusted to 10 with 50% ...
Embodiment 2
[0088] In above-mentioned Example 1, except using powdered cellulose (manufactured by Nippon Paper Co., Ltd., trade name "KC Flock W-100") 20g instead of cotton cloth (unbleached cotton fabric), metal chelate is obtained in the same way as in Example 1. 30.0 g of formable fibers (chelate fiber B) (50 mass % of substitution rate). The same adsorption experiment was carried out using the obtained chelate fiber B, and it was confirmed that 1.2 mmol of copper was captured per 1 g of the chelate fiber B.
Embodiment 3
[0090] A commercially available cartridge filter (manufactured by Advantec Toyo Co., Ltd., trade name "TCW-1-CSS": nominal pore size 1 μm), which is wound with cotton spun yarn on a core material made of stainless steel, is installed in a polypropylene 2.5 g of ammonium ferrous sulfate was dissolved in 10 liters of distilled water in a shell (manufactured by Advantec Toyo Co., Ltd., trade name "1PP-1-FS-000"), and the solution was circulated at a flow rate of 15 liters / minute with a circulation pump. Circulate 20° C. for 15 minutes, drain the liquid, and wash with 5 liters of distilled water in the same cycle.
[0091] Next, 60 g of glycidyl methacrylate, 3.0 g of a nonionic surfactant (manufactured by NOF Corporation, trade name "Nonionic OT-221"), 6.5 g of 31% hydrogen peroxide, and 2.5 g of thiourea dioxide were dissolved in In 8 liters of distilled water, the solution was also circulated at 60° C. for 20 hours to graft glycidyl methacrylate into the molecules of cotton spu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com