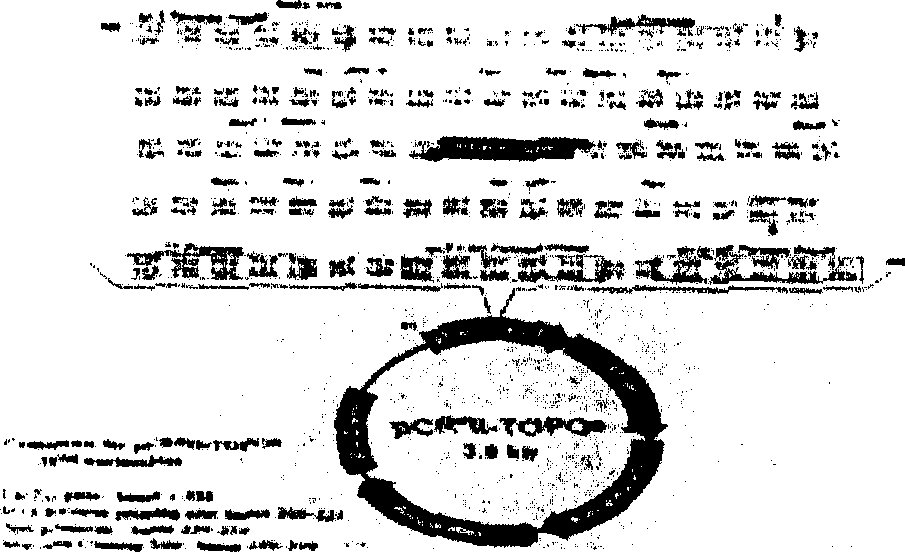Expression of recombinant human quick skeletal muscle type troponin C in colibacillus and its application for resisting tumor
A technology of troponin and recombinant plasmids, which is applied in the direction of anti-tumor drugs, applications, peptide/protein components, etc., and can solve the problems of difficulty in direct separation and purification, and failure to meet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The present invention will be further described below by way of examples. Example 1: Cloning and expression of the gene encoding human troponin TnC Primer design and synthesis
[0021] According to the known cDNA sequence of human troponin TnC, two primers were designed and synthesized, the sequences of which are as follows: 5'-end primer: 5'-AAC AGA GGA GGA GTC CCG GTC ACC AGC-3'3'-end primer: 5'-CTC CTT ACT GCA CGC CCT CCA TCA T-3′PCR
[0022] Using the human breast cDNA library as a template, a human troponin C (TnC) cDNA fragment with a length of 540 base pairs was amplified by PCR using the above two primers. The PCR reaction conditions are: 1 microgram of human breast cDNA library, 0.5 micrograms of each of the above two primers, 2 microliters of 10mM dNTPs, 10 microliters of 10×PCR buffer, 2.5 units of Taq DNA polymerase, and water to a final volume of 100 microliters. The PCR reaction was carried out in the PCR instrument according to the following temperature...
Embodiment 2
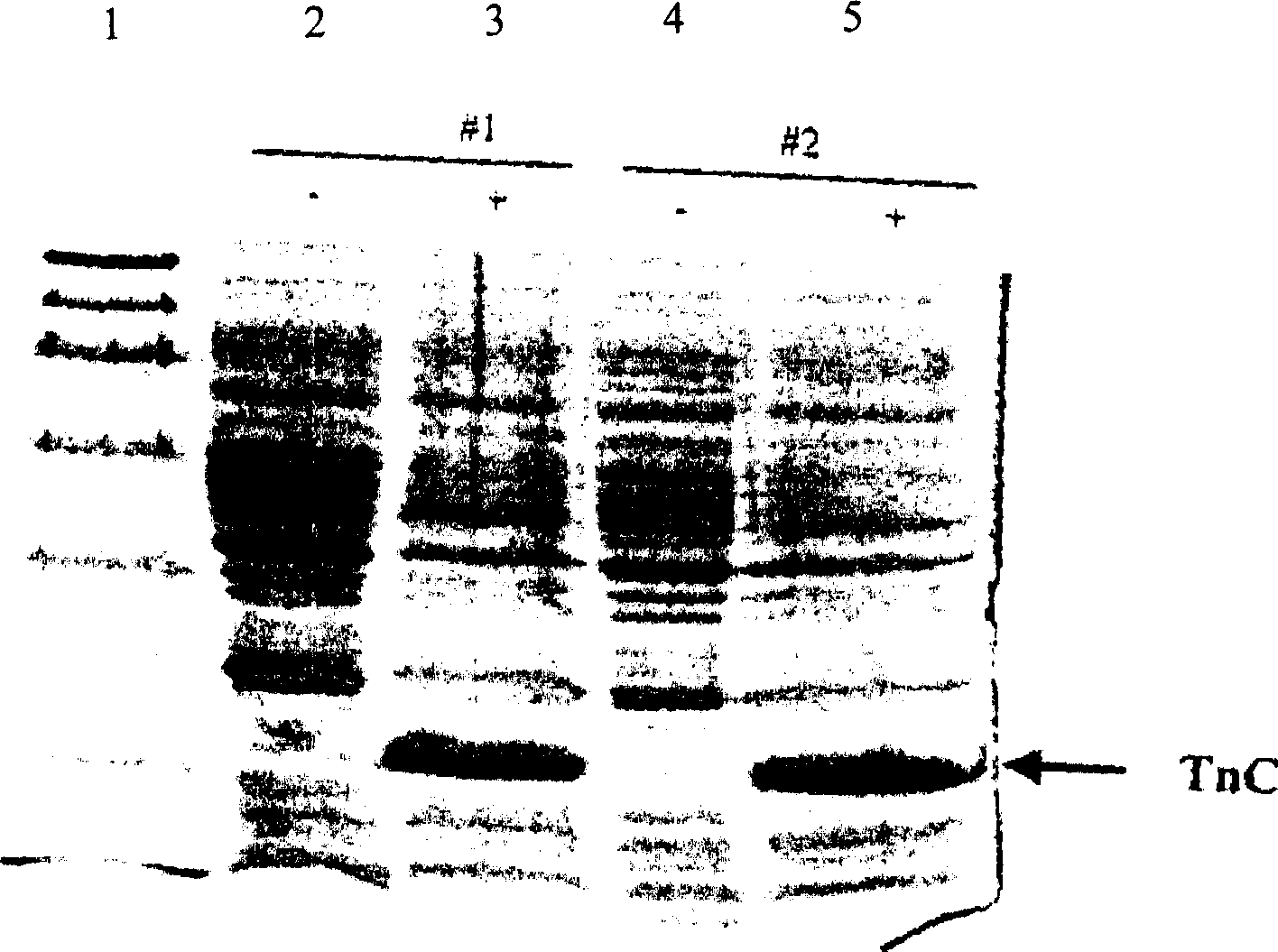
[0029] The pET3b-TnC expression plasmid was transformed into Escherichia coli BL21(DE3)pLysS, and the transformants with resistance to ampicillin and chloramphenicol were screened. The expression products were analyzed by SDS-PAGE ( image 3 ) analysis, it reached more than 30% of E. coli stainable protein, and the expression product was almost all in the form of soluble protein ( Figure 4 ). The engineered bacterium was named E.coli BL21(DE3)pLysS / pET3b-TnC, and the strain has been preserved in the China Type Culture Collection Center of Wuhan University with the number CCTCC No.M202028. Example 2: Expression and Purification of Human Troponin C Bacterial Culture and IPTG Induction
[0030] Inoculate a single fresh Escherichia coli engineered bacterium (E.coli BL21(DE3)pLysS / pET3b-TnC) into LB medium containing 200 μg / ml ampicillin and 34 μg / ml chloramphenicol, shake at 37°C, and culture overnight. The next day, it was diluted 1:100, and an appropriate amount of overnight...
Embodiment 3
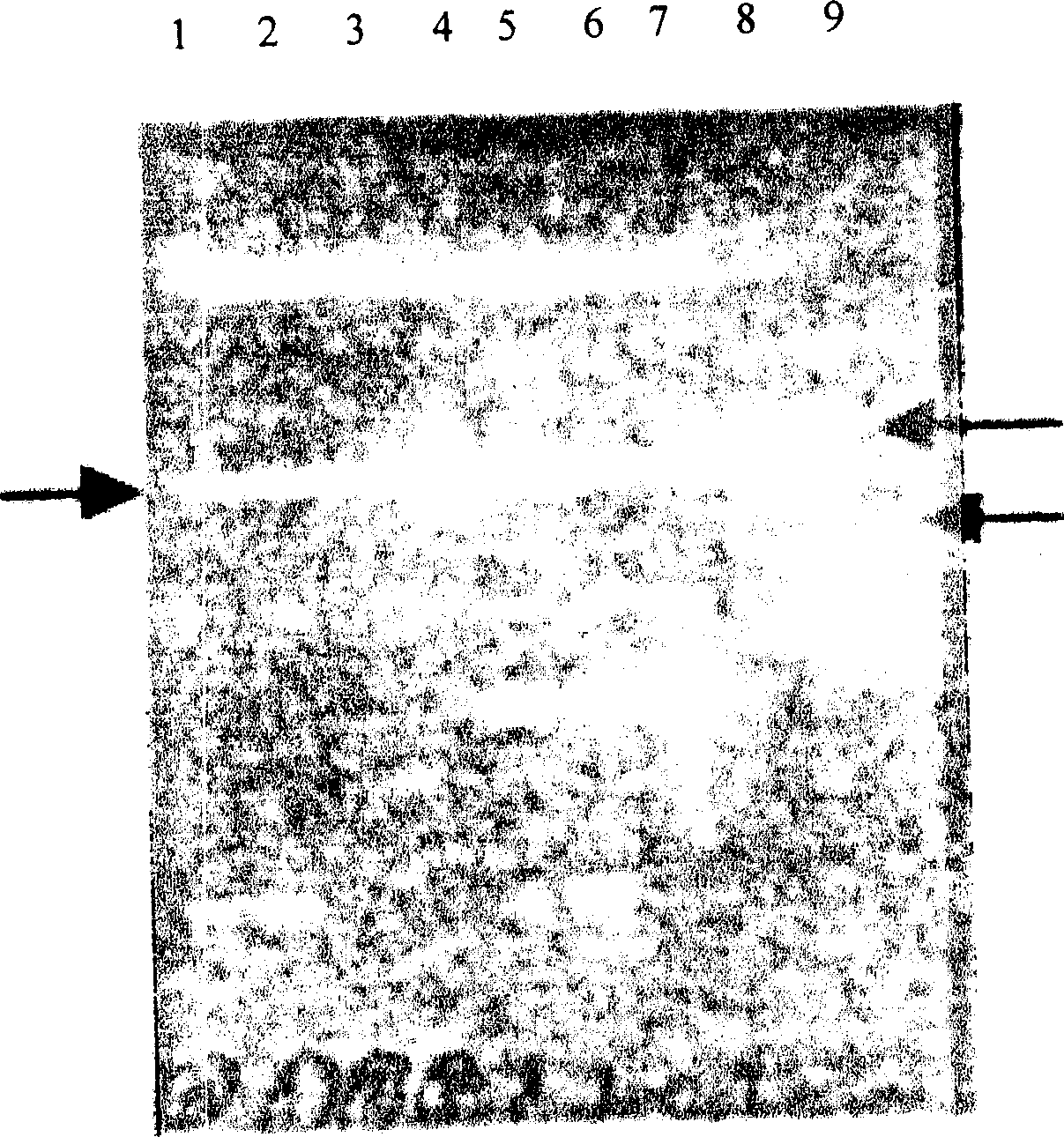
[0033] Equilibrate the column with PBS, load the above product peak solution, elute with PBS, collect in separate tubes, detect by SDS-PAGE, and finally combine and collect the product peaks. see results Image 6 , the grayscale scan of the SDS-DAGE spectrum of the purified protein showed a purity greater than 95%. Embodiment 3: biological activity detection: in vitro activity detection
[0034] Inhibitory effect of recombinant human troponin TnC on the growth of human umbilical vein endothelial cells in vitro. Human umbilical vein endothelial cells (HUV-EC, purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences) were mixed with 2×10 3 The density of the cell / well is placed on a 96-well plate for culture, and the culture medium is DMEM medium (containing growth factor bFGF 3ng / ml) containing 15% fetal bovine serum, cultured in a 5% CO2 incubator for 24 hours to allow it to adhere to the wall, and then add medicine. After adding diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com