Process for producing optically active sulfoxide devivative'
A compound and substituent technology, applied in the field of optically active sulfoxide derivatives, can solve the problems of low yield and low optical purity of sulfoxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
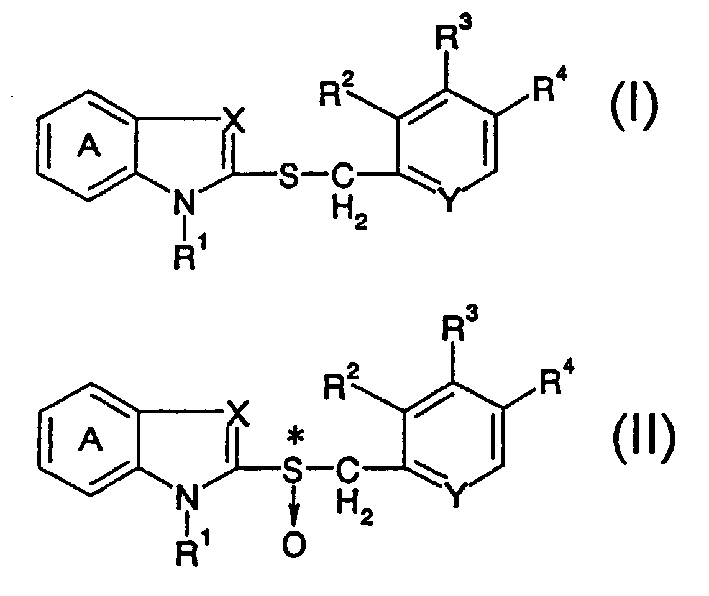
[0108] The preparation of embodiment 1 (R)-2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]benzimidazole (1) Under a nitrogen protection atmosphere, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]thio]benzimidazole (50.0 g, 0.14 mol, containing 16.7 mg of water), toluene (250 ml), water (283 mg, 0.016 mol, total moisture content 0.017 mol) and (+)-diethyl tartrate (10.6 ml, 0.062 mol) were mixed , and the mixture was stirred at 50-55°C for 30 minutes. Under a nitrogen protective atmosphere, titanium(IV) isopropoxide (8.29 mL, 0.028 mol) was added, and the mixture was stirred at 50-55°C for 1 hour. Under a nitrogen protective atmosphere and cooling, diisopropylethylamine (8.13 ml, 0.047 mol) was added to the resulting mixture, and then cumene hydroperoxide (76.50 ml, content 82% , 0.43 mol). The mixture was stirred at -10 to 10°C for 4.5 hours.
[0109] Analysis of the reaction mixture by high performance liquid chromatography (condition (A)) rev...
Embodiment 2
[0112] Example 2 Preparation of (R)-2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]benzimidazole (1) Under nitrogen protection gas flow, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]thio]benzimidazole (900 g, 2.55 mol, containing 80 mg of water), toluene (4500 ml), water (5.4 g, 0.300 mol, total moisture content 0.304 mol) and (+)-diethyl tartrate (192 ml, 1.12 mol) were mixed , and the mixture was stirred at 50-56°C for 30 minutes. Under nitrogen gas flow, titanium(IV) isopropoxide (149 ml, 0.505 mol) was added, and the mixture was stirred at 53-56°C for 1 hour. Under nitrogen protection flow, the mixture was cooled to room temperature, diisopropylethylamine (147 ml, 0.844 mol) was added to the resulting mixture, and then cumene hydroperoxide (1380 ml, content 82 %, 7.70 moles). The mixture was stirred at -5 to 5°C for 2 hours.
[0113] The resulting reaction mixture was analyzed by high performance liquid chromatography (condition (A)). As...
Embodiment 3
[0115] Example 3 Preparation of (R)-2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]benzimidazole
[0116] 2-[[[3-Methyl 4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfanyl]benzimidazole (25.0 g, 0.071 mole, containing 13.4 mg water), toluene (122 mL), water (137 mg, 0.0076 mol, total moisture content 0.0083 mol) and (+)-diethyl tartrate (5.32 mL, 0.031 mol). To the mixture was added titanium(IV) isopropoxide (4.15 ml, 0.014 mol) at 50-60°C, and the mixture was stirred at 50-55°C for 1 hour. To the resulting mixture was added diisopropylethylamine (4.07 ml, 0.023 mol) at room temperature, and then cumene hydroperoxide (38.2 ml, content 82%, 0.22 mol) was added at -5 to 5°C. The mixture was stirred at -5 to 5°C for 1.5 hours.
[0117] The resulting reaction mixture was analyzed by high performance liquid chromatography (condition (A)). As a result, it was found that 0.60% of thioether and 1.76% of sulfone existed as analogs in the reaction mixture, and no o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com