Rare earth dithiocarbamate vulcanization acalerator and its preparation method
A technology of dithiocarbamic acid and vulcanization accelerator is applied in the field of rare earth complexes, which can solve the problems of toxicity, poor vulcanization stability, easy scorch, etc., and achieve the effects of mild reaction conditions, good performance and easy acquisition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
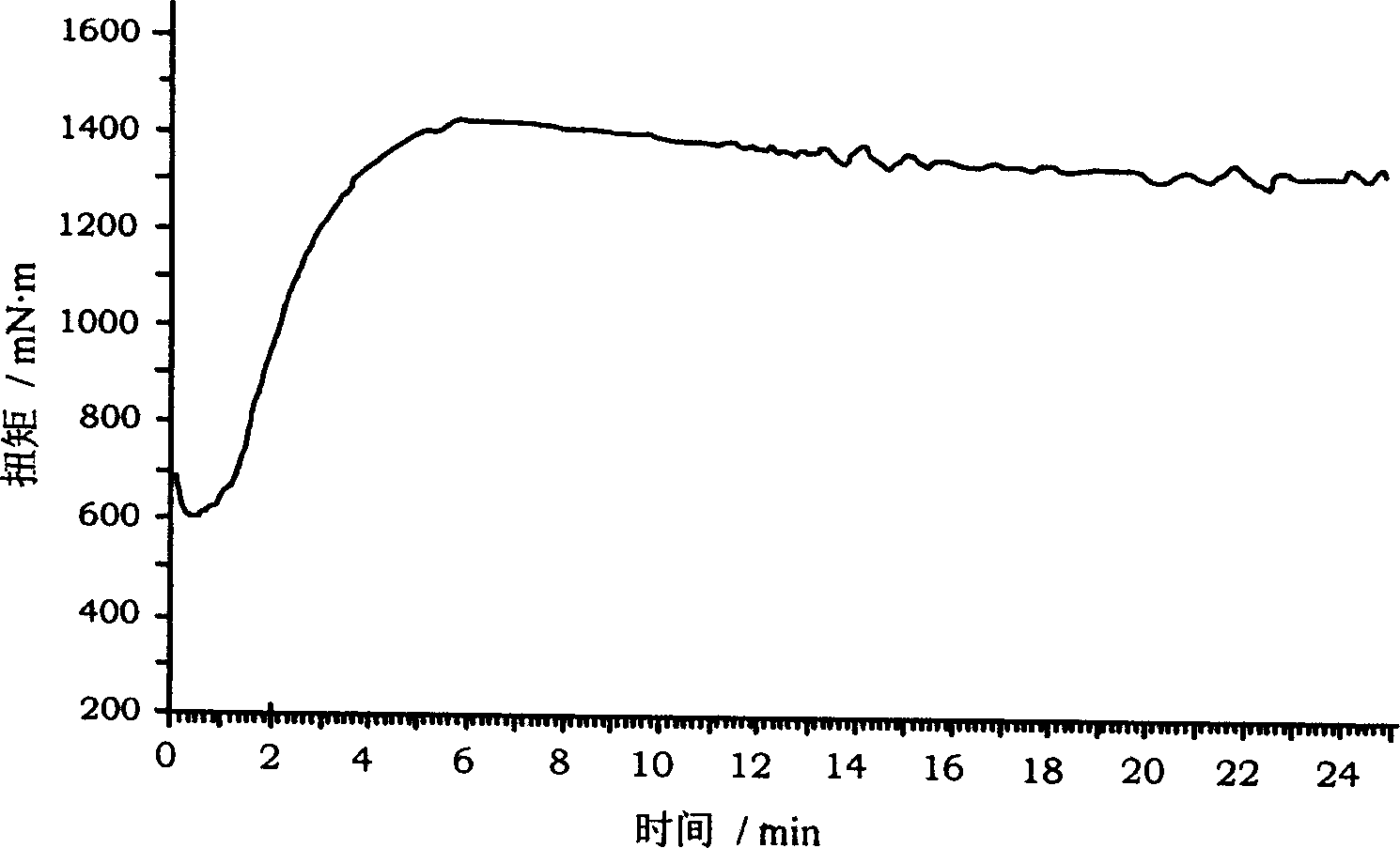
Examples
Embodiment 1
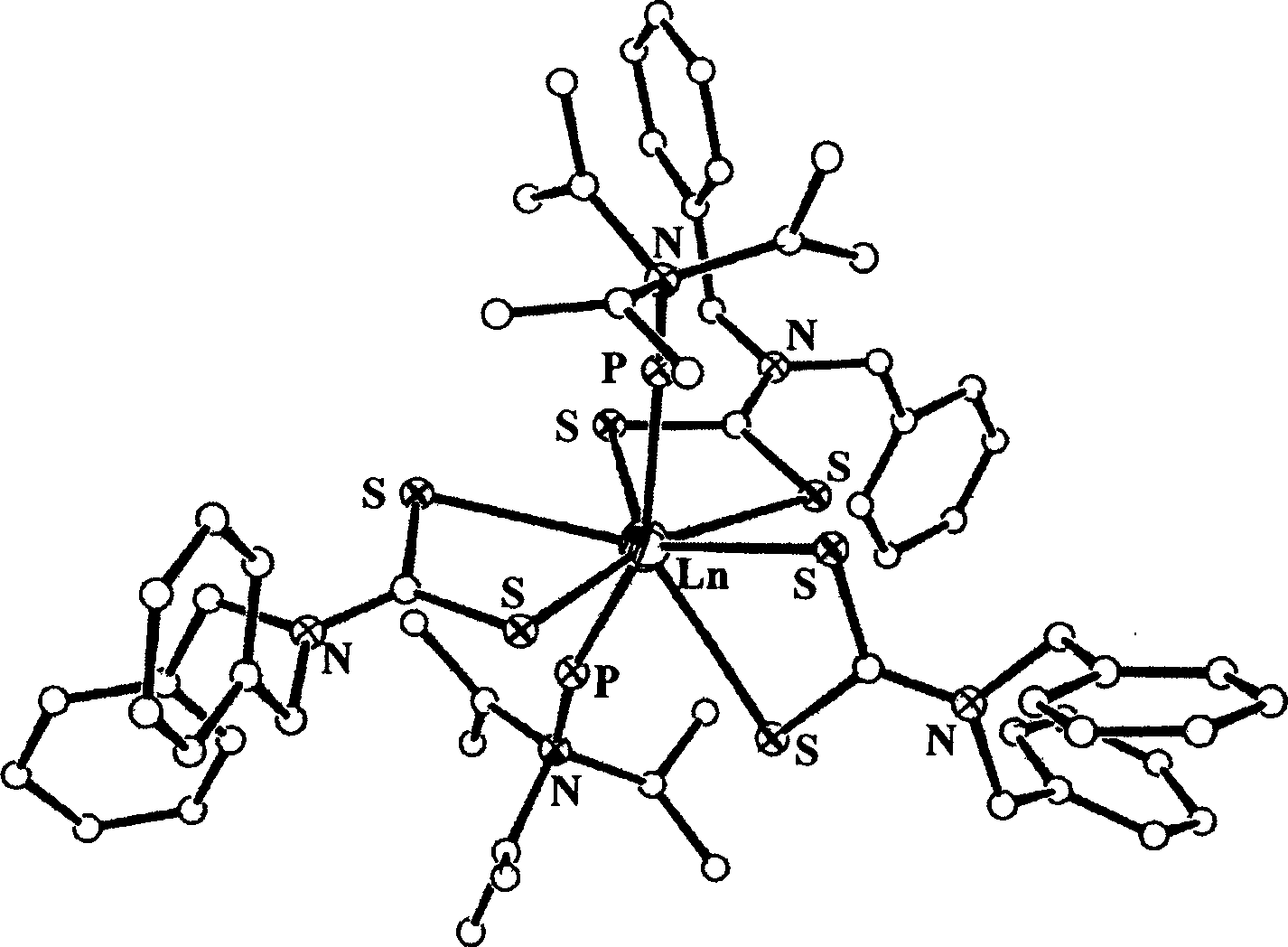
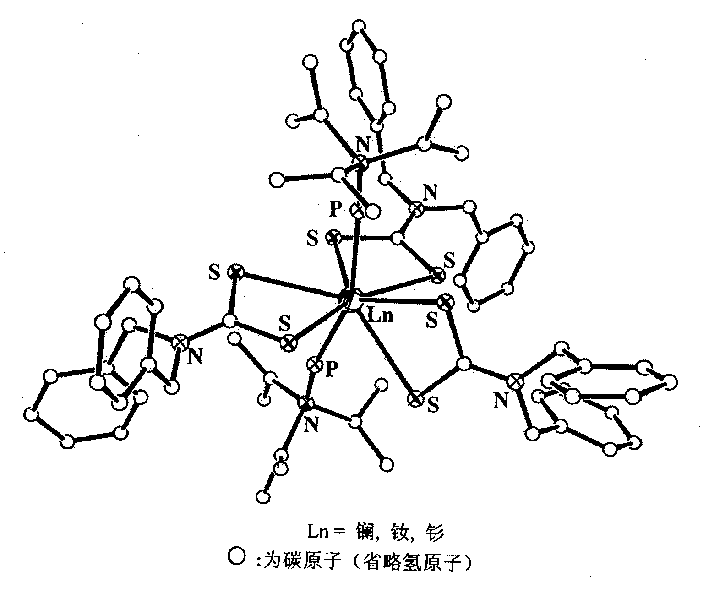
[0023] (1) In a three-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, using THF as a solvent, add 1.00 mol of dibenzylamine, 1.12 mol of NaOH and 1.20 mol of CS in sequence 2 , control the reaction temperature at 50±2°C, stir the reaction for 2.5h; evaporate the solvent to obtain a white solid, wash with ether three times to obtain 280.5g of the white ligand.
[0024] (2) In a 500mL Erlenmeyer flask, dissolve 0.60mol of the ligand obtained in reaction (1) into 250mL of water, add 0.20mol of solid lanthanum chloride under stirring at 60°C, continue stirring for 5h, and filter to obtain a white powder .
[0025] (3) Dissolve the product of step (2) in acetone at normal temperature, and slowly drop 0.43mol of hexamethylene phosphoramide under stirring conditions; react for 2h, and naturally volatilize the solvent to obtain the lanthanum rare earth complex of the present invention thing.
Embodiment 2
[0027] (1) In a three-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, using tetrahydrofuran as a solvent, add 1.00 mol of dibenzylamine, 1.10 mol of NaOH and 1.40 mol of CS 2 , control the reaction temperature at 50±2°C, stir the reaction for 3h; evaporate the solvent to obtain a white solid, wash with ether 3 times to obtain 279.4g of the white ligand.
[0028] (2) In a 500mL Erlenmeyer flask, dissolve 0.63mol of the ligand obtained in reaction (1) in 250mL of water, add 0.22mol of solid neodymium chloride under stirring at 60°C, continue stirring for 4 hours, and filter to obtain light blue color powder.
[0029] (3) Dissolve the product of step (2) in acetone at normal temperature, slowly drop into 0.45mol of hexamethylene phosphoramide under stirring conditions; react for 1.9h, and naturally volatilize the solvent to obtain neodymium rare earth of the present invention Complexes.
Embodiment 3
[0031] (1) In a three-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, using tetrahydrofuran as a solvent, add 1.00 mol of dibenzylamine, 1.20 mol of NaOH and 1.32 mol of CS 2 , control the reaction temperature at 50±2°C, stir the reaction for 2h; evaporate the solvent to obtain a white solid, wash with ether 3 times to obtain 281.5g of the white ligand.
[0032] (2) In a 500mL Erlenmeyer flask, take 0.66mol of the ligand obtained in reaction (1) and dissolve it in 250mL of water, add 0.24mol of solid samarium chloride under stirring at 70°C, continue stirring for 5h, and filter to obtain pink powder.
[0033] (3) Dissolve the product of step (2) in acetone at normal temperature, slowly drop into 0.484mol hexamethylene phosphoramide under stirring condition; react for 2.1h, and naturally volatilize the solvent to obtain the samarium rare earth of the present invention Complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com