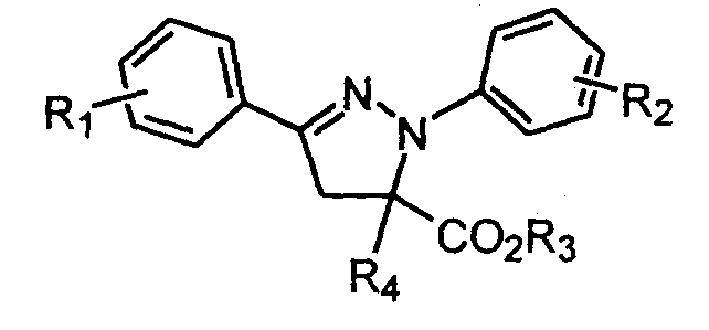
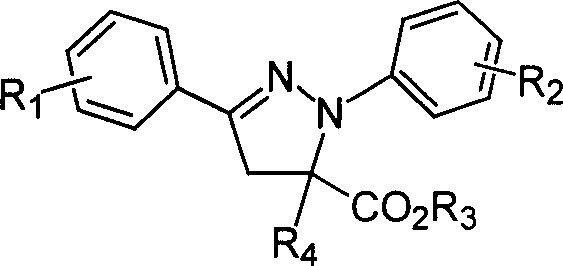
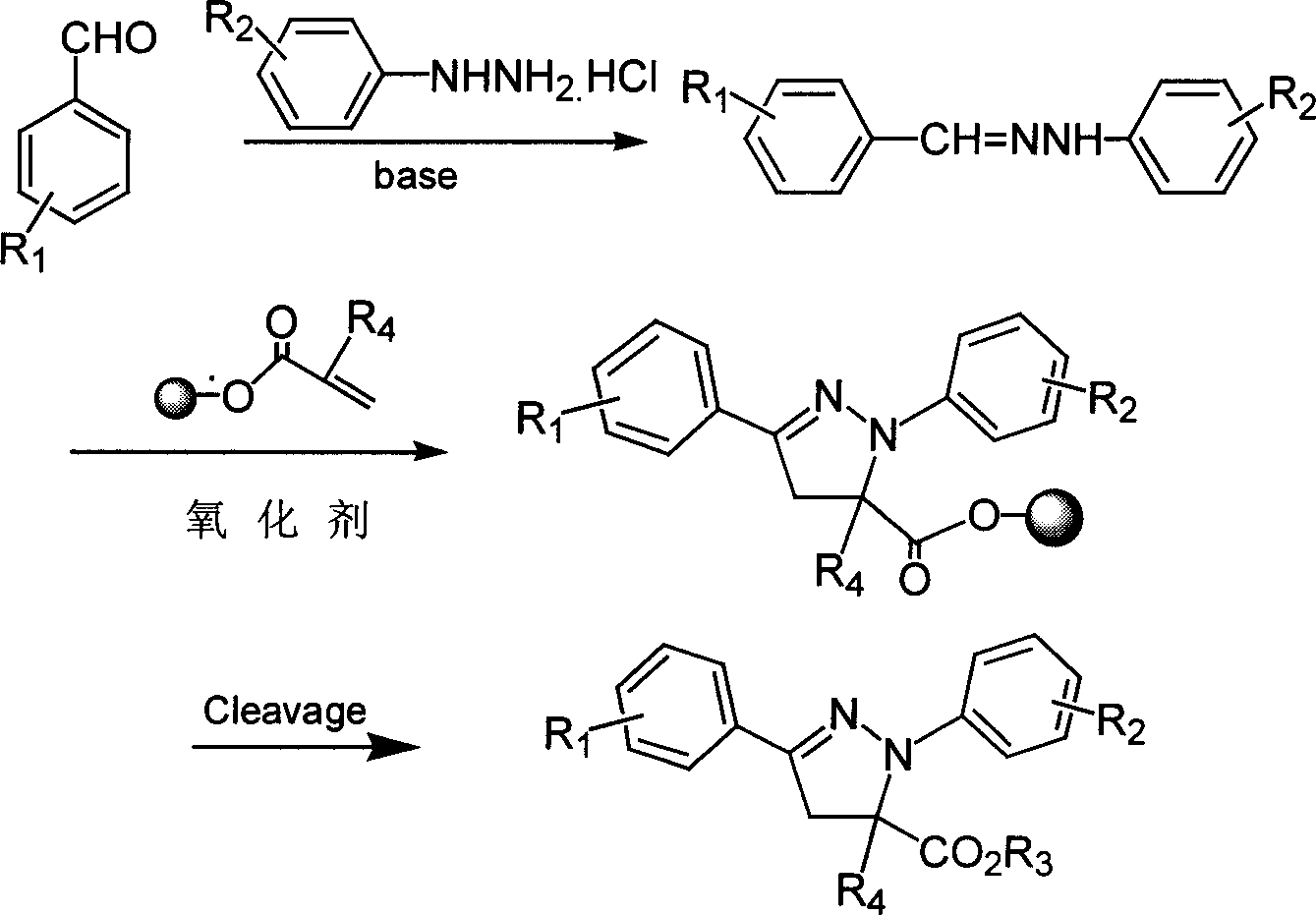
1,3-aryl group substituted pyrazoline whose 5 position contains ester gruop and its liquid phase synthesis method
A kind of pyrazoline liquid, pyrazoline technology, applied in 1 field, can solve the problems such as can not be directly fully utilized, unsatisfactory yield, time-consuming and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step 1: Dissolve 15g of polyethylene glycol (PEG4000) with a molecular weight of 4000 in 60ml of dichloromethane, add 4.16ml of trioctylamine, cool in an ice bath, and slowly add 2.45ml of acryloyl chloride dropwise over 2 hours. After the dropwise addition, the ice bath was removed, and the reaction was carried out at room temperature for 24 hours. After the reaction is complete, add 500ml of anhydrous ether and shake until the solution becomes turbid, freeze for 30 minutes, precipitate crystals, filter with suction, wash with anhydrous ether three times, put it in a desiccator, and vacuum dry overnight to obtain 14.9g of acrylate supported on PEG4000.
[0025] Step 2: Under nitrogen protection, add 185 mg of phenylhydrazine hydrochloride, 8 ml of methanol, 0.5 ml of trioctylamine, 133 mg of benzaldehyde, and stir at room temperature for 30 minutes in a 10 ml small flask; then add 281 mg of chloramine T and PEG4000 support 0.5g of acrylate, temperature controlled at 40...
Embodiment 2
[0029] The reaction steps are the same as in Example 1, except that 3.0ml of methacryloyl chloride is used in step 1; the product obtained is 1-phenyl-3-phenyl-5-methyl-5-formic acid methyl pyridyl oxazoline. The purity is 100%, and the yield is 91%.
[0030] 1 H-NMR (500MHZ, CDCl 3 )δ=1.64(s, 3H), 3.31(d, J=16.6Hz, 1H), 3.71(d, J=16.6Hz, 1H), 3.77(s, 3H), 6.88(t, 1H), 7.11( d, J=8.3Hz, 2H), 7.25(d, J=7.3Hz, 2H), 7.40(m, 3H), 7.70(d, J=7.3Hz, 2H).
Embodiment 3
[0032] The reaction steps are the same as in Example 1, except that what is used in step 1 is 3.0ml methacryloyl chloride; the solvent used for crystallization in step 2 is 50ml isopropanol; the aromatic aldehyde added in step 2 is p-methoxy 164 mg of benzaldehyde. The product obtained was 1-phenyl 3-p-methoxyphenyl 5-methyl-5-methyl carboxylate pyrazoline. The purity is 98%, and the yield is 90%.
[0033] 1 H-NMR (500MHZ, CDCl 3 )δ=1.62(s, 3H), 3.27(d, J=16.5Hz, 1H), 3.69(d, J=16.5Hz, 1H), 3.76(s, 3H), 3.84(s, 3H), 6.86( t, 1H), 6.91(d, J=8.7Hz, 2H), 7.10(d, J=7.0Hz, 2H), 7.23(m, 2H), 7.65(d, J=7.0Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com