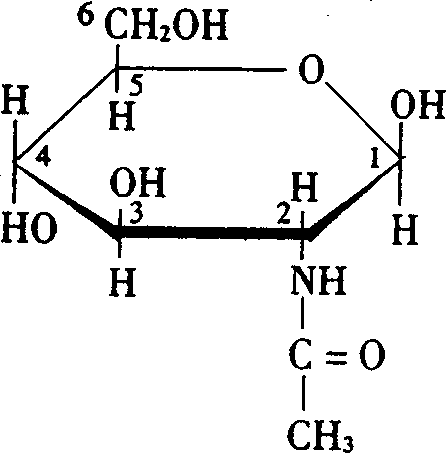
Application of N-acetylglucosamine in the preparation of medicine for treating viscera injure due to toxication from poison and medicine
一种乙酰氨基、葡萄糖的技术,应用在药物组合、抗毒剂、医药配方等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1. The wave-promoting test of the compound of formula (I)
[0021] 1. Test materials and methods:
[0022] 1.1 Sample: pure compound of formula (I).
[0023] 1.2 Test material:
[0024] Bacteria: Proteus mirabilis, should meet the following biochemical reaction characteristics: motility (+), urease (+), lactose (-), glucose (+), H 2 S (-), phenylalanine deaminase (+).
[0025] Medium: modified LB medium (composition: 1% tryptone, 0.5% yeast extract, 1% sodium chloride, 0.1% glucose, 0.002% TTC, pH7.2-7.4).
[0026] 1.3 Test method:
[0027] Control sample: plant Proteus mirabilis in the center of the LB plate and culture at 37°C for 9 hours;
[0028] Test sample: Add the compound of formula (I) at a final concentration of 0.5% to the LB plate, plant Proteus mirabilis in the same way, and culture at 37° C. for 9 hours.
[0029] 2. Test results and evaluation:
[0030] In the control sample, there are concentric rings that continuously expand outwards at...
Embodiment 2
[0032] The toxicological test of embodiment 2. formula (I) compound
[0033] Carry out the toxicology test of formula (I) compound, comprise:
[0034] 1. Acute toxicology test: including oral, intravenous injection and maximum dose administration test;
[0035] 2. Ames test;
[0036] 3. Mouse bone marrow cell micronucleus test;
[0037] 4. Mouse sperm teratogenic test;
[0038] 5. Chromosomal aberration test of mouse testis;
[0039] 6. Chronic lethal test;
[0040] 7. Subchronic toxicity (90-day feeding) test;
[0041] 8. Traditional teratogenicity test;
[0042] Test conclusion shows: when formula (I) compound acute toxicity test dose exceeds 2g / kg, acute poisoning reaction does not yet occur; In long-term toxicity test, the highest dose has reached 1g / kg, through four weeks test observation, no toxic reaction occurs; In the reproduction test, mice were fed from a conventional dose of 7 mg / kg, and after three passages, it was proved that the compound of formula (I) had ...
Embodiment 3
[0043] Embodiment 3. Animal test
[0044] (1) Methanol poisoning test:
[0045] Thirty Kunming mice were used for the experiment, and they were divided into an experimental group and an experimental group, with 15 mice in each group. In addition, 15 mice were used as controls. Each mouse was orally fed with methanol at a dose of 0.2ml / 20g body weight. In the control group, no treatment measures were taken, and the mice showed delirium, bumping around, and blurred vision. As time went on, in about 2 hours, 13 mice became blind in both eyes and limbs, and 10 mice died one after another, and the blindness symptoms of the other 3 mice did not improve. After the mice in the experimental group were fed with methanol, they were given an aqueous solution of N-acetylglucosamine with a concentration of 0.1g / ml by intraperitoneal injection immediately. 5 rats died one after another), and 2 rats had obvious neuropsychiatric symptoms. After feeding methanol, the mice in the second gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com