Synthesis of poly-alpha olefin and use thereof.
A technology of olefins and oligomers, which is applied in the field of lubricant compositions and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
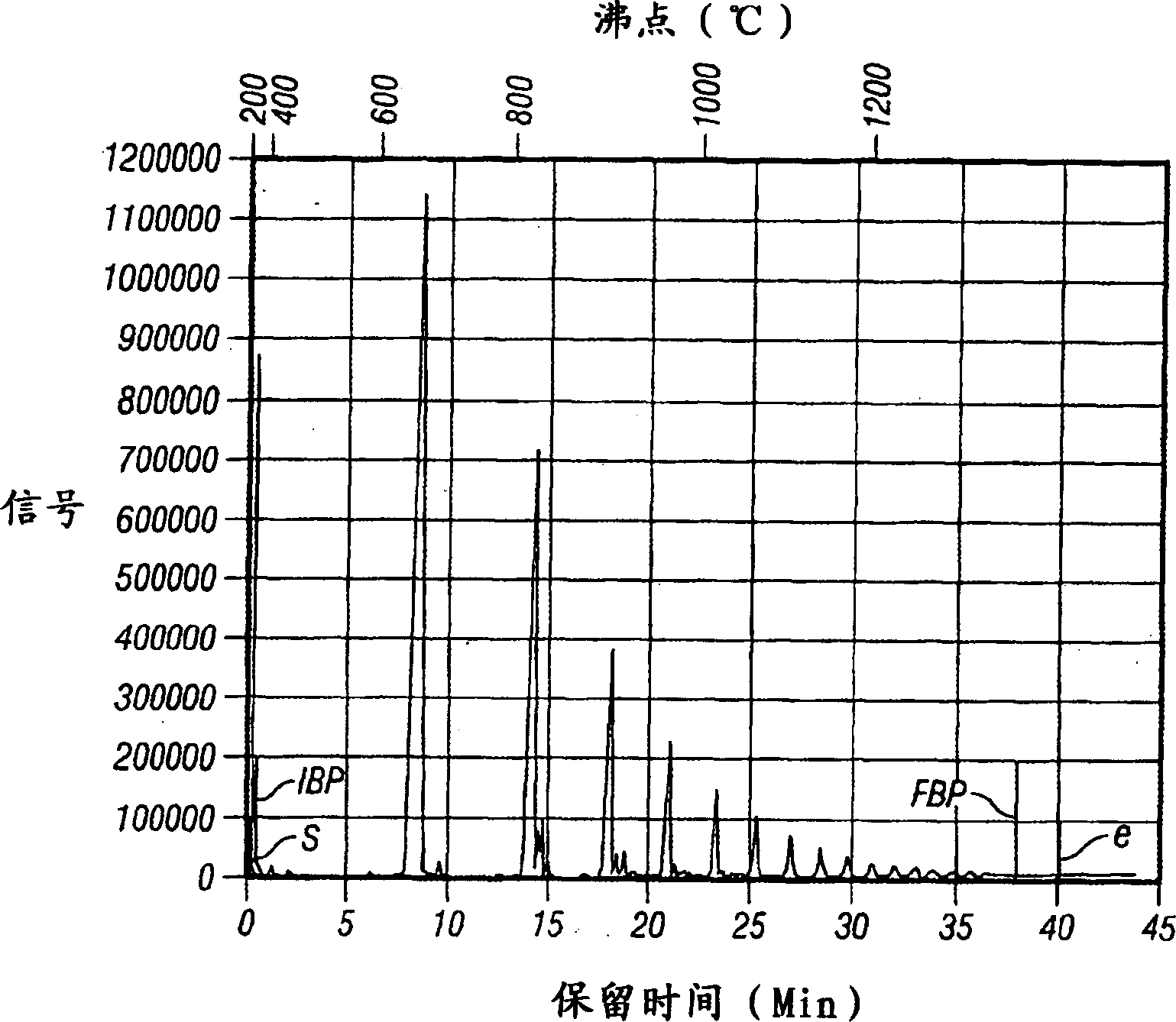
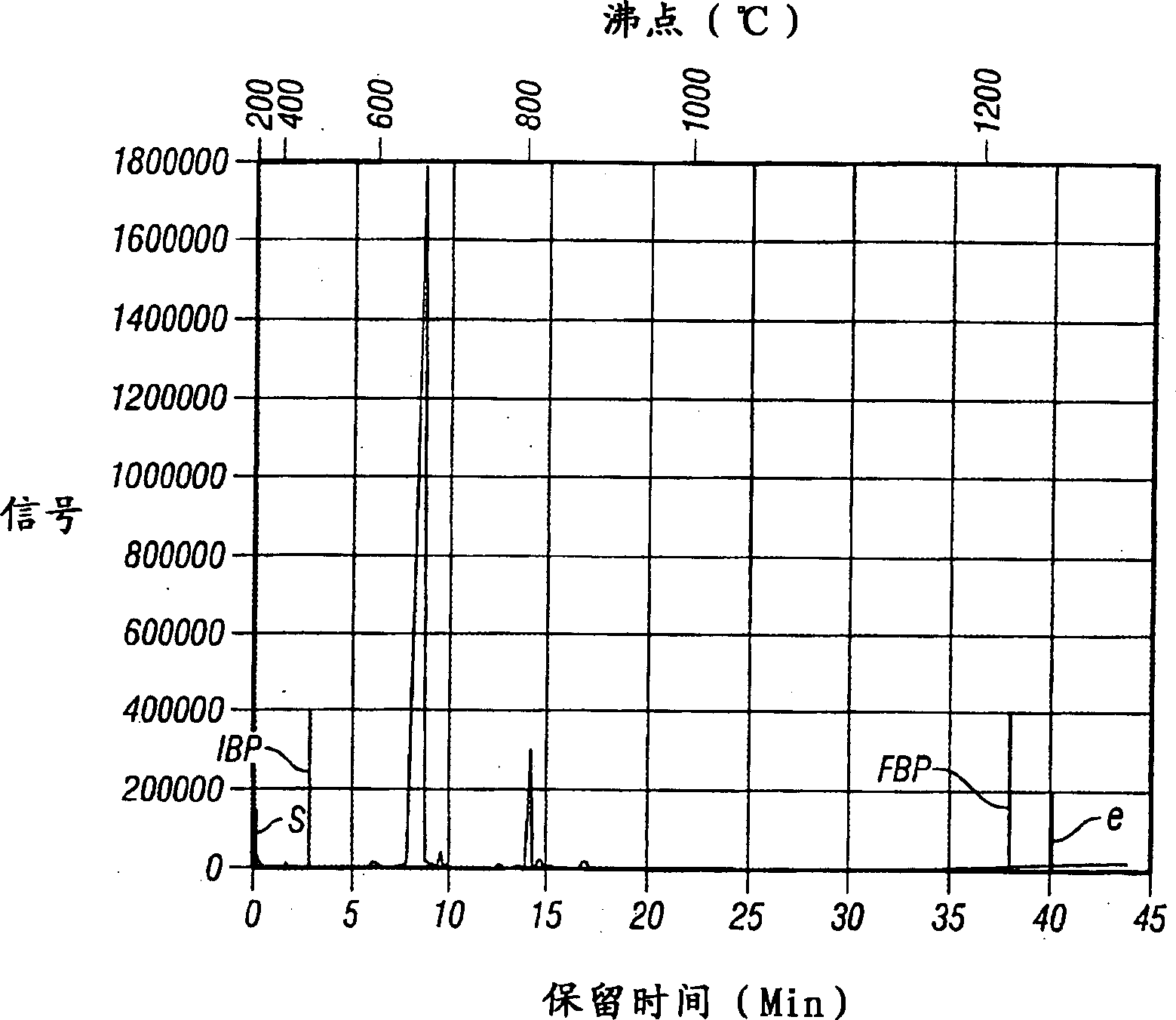
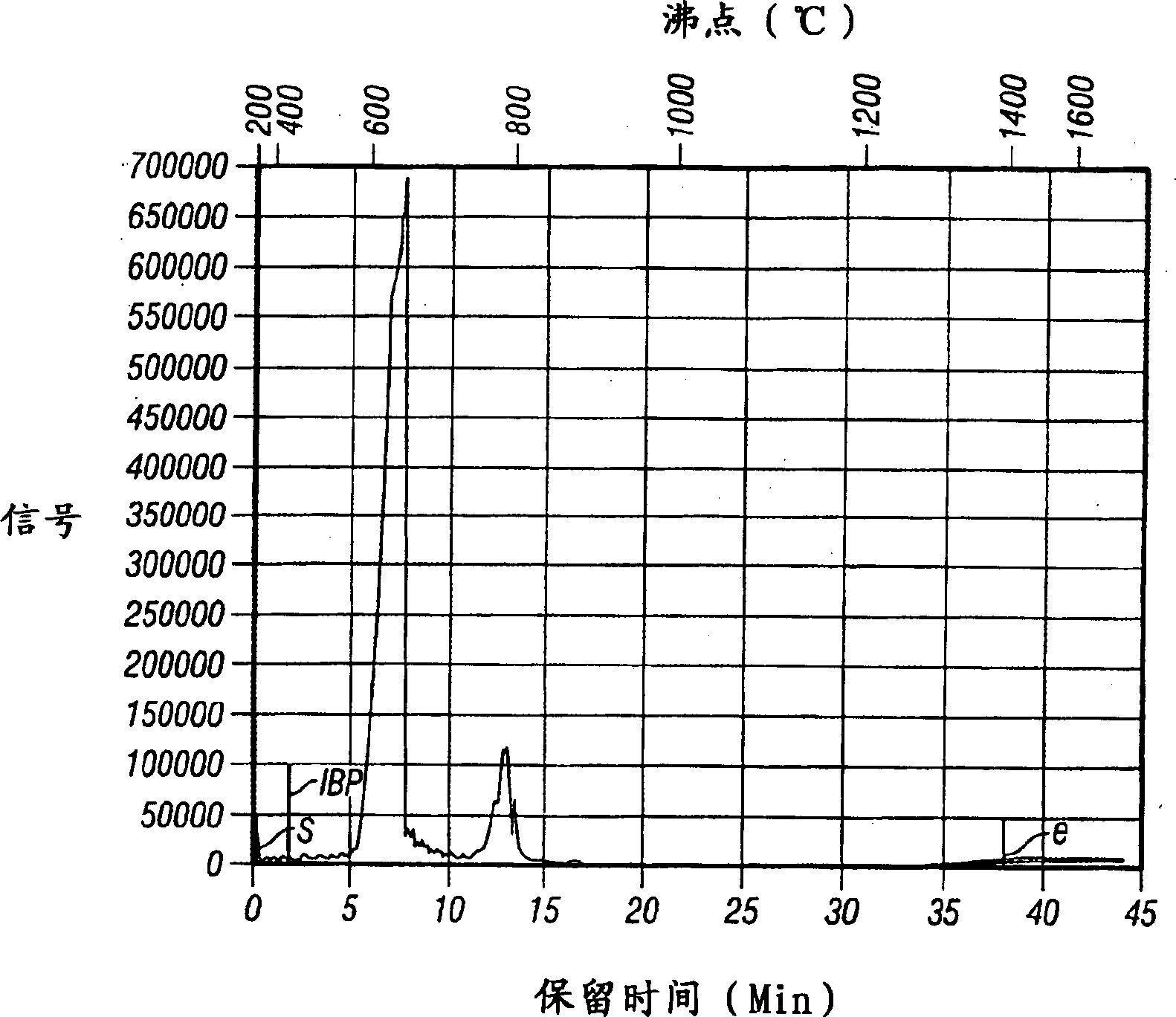
Image
Examples
Embodiment 1
[0085] A 120ml pressure reaction vial with a magnetic stirring bar was completely evacuated and then blown with argon. Then, 10 ml of anhydrous toluene (distilled with potassium treatment) and 10 ml of 1-tetradecene (dried over 5A molecular sieves) were added to the bottle. At 40° C., 4 ml of a 3.3 M methylaluminoxane solution in toluene was added to the reaction flask and stirred for 15 minutes. Then inject 4ml 6.2×10 into the bottle - 3 M's dichlorobis(cyclopentadienyl) zirconium in toluene to start the reaction. The temperature of the reaction system was controlled within ±1°C by a constant temperature bath. After 1 hour, 50 ml of 10% aqueous HCl was added to the reaction flask to stop the reaction, and the resulting mixture was stirred for 2 hours. The organic layer was then separated and further washed twice with 50 ml of deionized water. Subsequently, the toluene solvent was removed from the organic layer in a rotary evaporator. Analysis of the product mixture by hi...
Embodiment 2
[0087] The procedure was essentially the same as in Example 1, except that the reaction was carried out at 60°C. The oligomer yield was 86%. The oligomer contains about 60% dimers, 23% trimers, 8% tetramers, 4% pentamers and 5% higher oligomers.
Embodiment 3
[0089] The procedure was essentially the same as in Example 1, except that the reaction was carried out at 60°C and 2 ml of 3.3 M methylalumoxane in toluene was used. The oligomer yield was 87%. The oligomer contains approximately 68% dimers, 20% trimers, 7% tetramers, 3% pentamers and 2% higher oligomers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| kinematic viscosity | aaaaa | aaaaa |
| kinematic viscosity | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com