Method for preparing terazosin hydrochloride of optical activity
A terazosin hydrochloride, optically active technology, applied in the field of organic synthesis, can solve the problems of low reaction selectivity, high toxicity of intermediates, difficult to obtain raw materials, etc., and achieves simple process conditions, good selectivity and easy synthesis reaction. The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
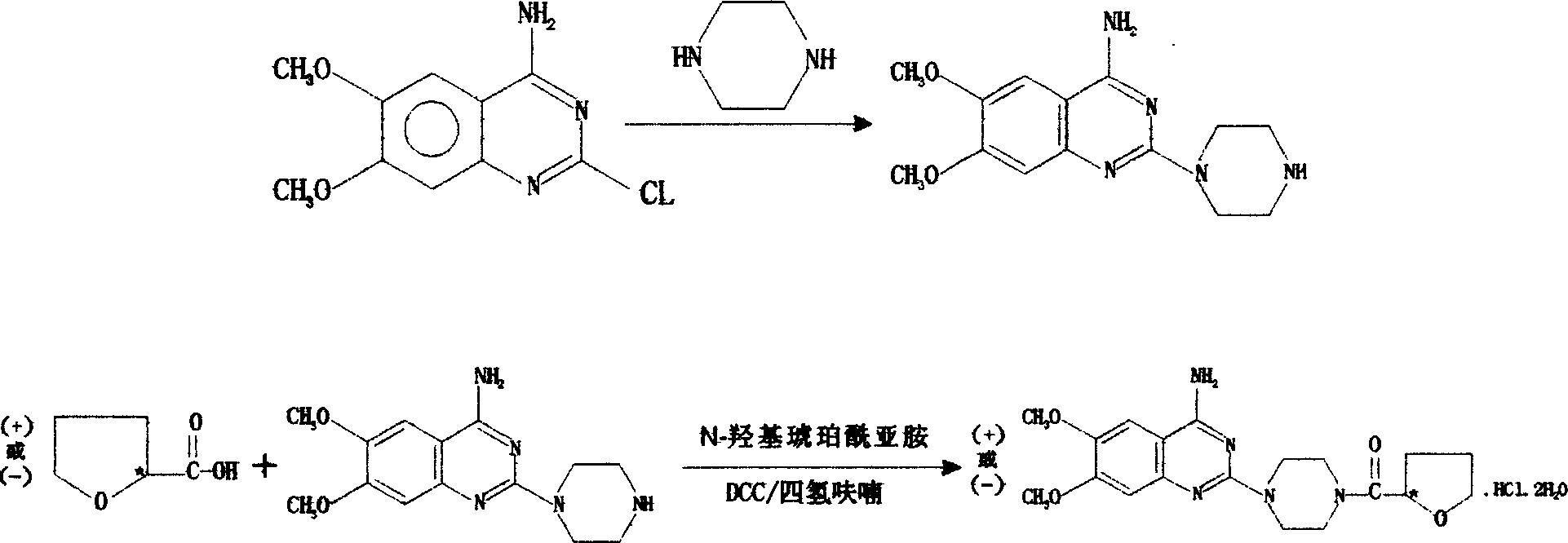
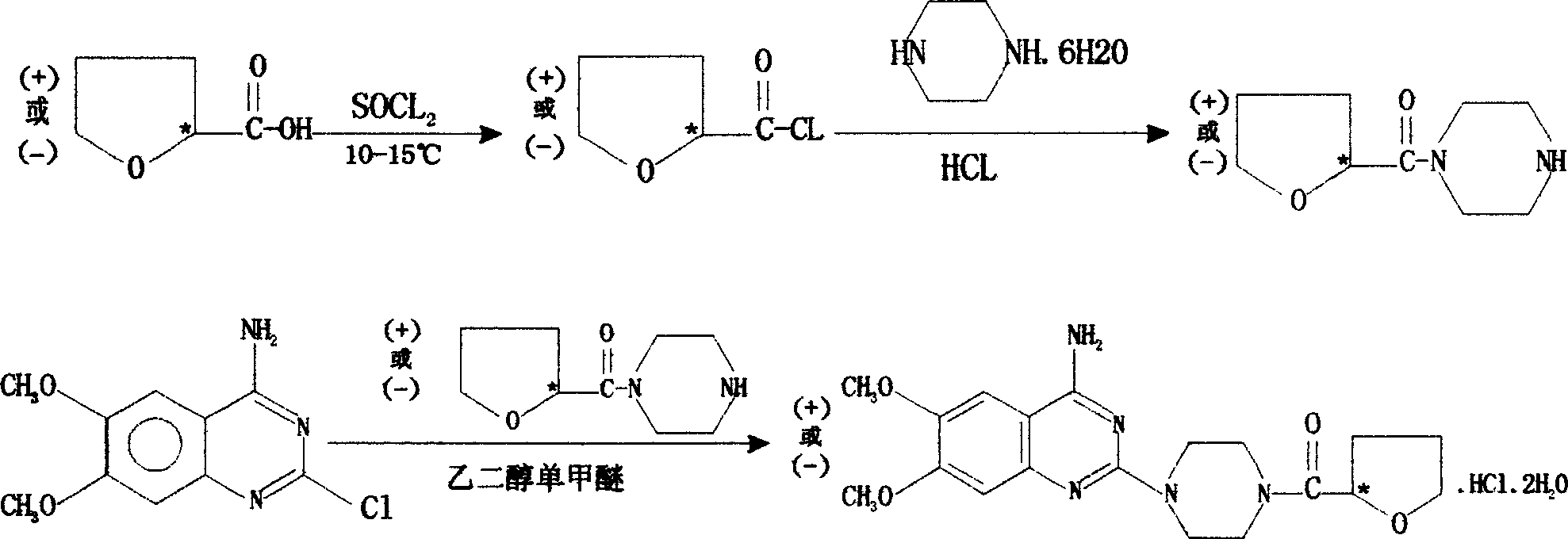
[0014] Preparation of R-(+)-N-(tetrahydro-2-furoyl)piperazine
[0015] R-(+)-tetrahydro-2-furoic acid 50ml ([α] 29℃ =+33.0°, C=1, CHCl 3 ) was added to a 2L three-necked flask, and SOCL was added dropwise at room temperature 2 60ml, the dropwise addition was completed, continued to stir for 4 hours, raised the temperature and refluxed for 1 hour, and distilled under reduced pressure to obtain 57.5g of R-(+)-tetrahydro-2-furoyl chloride.
[0016] Add 91.3g1 of piperazine hexahydrate to a 2L three-necked flask, adjust the pH value to 3.6 with about 85ml of concentrated hydrochloric acid, add 10ml of chloroform and stir the mixture to lower the temperature below 10°C, and add the obtained R- (+)-Tetrahydro-2-furoyl chloride, add anhydrous sodium acetate to adjust the pH of the reactant between 3.5 and 4.0 during the dropwise addition, after the dropwise addition is completed (3~3.5 hours), keep warm at 10~15°C React for 3 hours, adjust the pH of the reaction solution to 2.5 w...
Embodiment 2
[0018] Preparation of R-(+)-terazosin hydrochloride
[0019] 65.8g of 4-amino-2-chloro-6,7-dimethoxyquinazoline, R-(+)-N-(tetrahydro-2-furoyl)piperazine 60g and ethylene glycol monomethyl Add 200ml of ether into a 500ml three-necked flask in turn, pass N2↑ protection, control the temperature at 120-123°C, react for 2 hours, add 150ml of isopropanol and keep it at 65°C, stir and react for 1 hour, cool down, and adjust the pH to 2.5 with concentrated hydrochloric acid After stirring for 10 minutes, filter with suction, wash the filter cake twice with isopropanol, take out the filter cake, add 250ml of 95% ethanol, stir well, adjust the pH value to 8.5 with ammonia water, stir for 40 minutes, filter with suction, filter the cake, Distilled water 500ml, add concentrated hydrochloric acid at 10°C to adjust the pH value to 2.5, stir for 30 minutes, filter, adjust the pH value of the filtrate to 8.5 with ammonia water, keep warm at 40-45°C and stir for 30 minutes, cool to 10-15°C, su...
Embodiment 3
[0022] Preparation of S-(-)-N-(tetrahydro-2-furoyl)piperazine
[0023] The method for preparing R-(+)-N-(tetrahydro-2-furoyl)piperazine in Example 1 was used to prepare S-(-)-N-(tetrahydro-2-furoyl)piperazine. b.p.110-116℃ / 1.8Pa, n D 25.5℃ = 1.5188, [a] D 25.5℃ =-25.0° (C=1, H 2 O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com