Prawn leukasmus rhabdovirus WP486 gene and coded polypeptide thereof
A WP486, encoding technology, applied in the direction of viral peptides, genetic engineering, plant genetic improvement, etc., can solve the problem of less virus molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Cloning and sequencing of WP486 gene fragment
[0027] Using WSBV genomic DNA as a template, the WP486 gene was amplified with primers P1 and P2.
[0028] P1: 5′-CGT GGATCC GACACAGACGATTTTGAGCC-3' (SEQ ID No. 3);
[0029] P2: 5′-GA GCGGCCGC A GCTAGC TTGGCTGGACAATAAAT-3' (SEQ ID No. 4);
[0030] underlined part GGATCCis the BamH I restriction site, GCGGCCGC is the NotI restriction site, GCTAGC It is the NheI restriction site.
[0031] The PCR reaction conditions are:
[0032] 94°C 60 seconds
[0033] 55℃ for 60 seconds
[0034] 72°C for 1.5 minutes (30 cycles)
[0035] After the amplified fragment was purified by agarose gel electrophoresis, it was cloned into the prokaryotic expression plasmid vector pGEX4T-1 to obtain the recombinant expression plasmid pGEX-WP486, and then sequenced. See SEQ ID No.1 for the WP486 gene sequence obtained by sequencing.
[0036] The amino acid sequence of WP486 deduced according to the obtai...
Embodiment 2
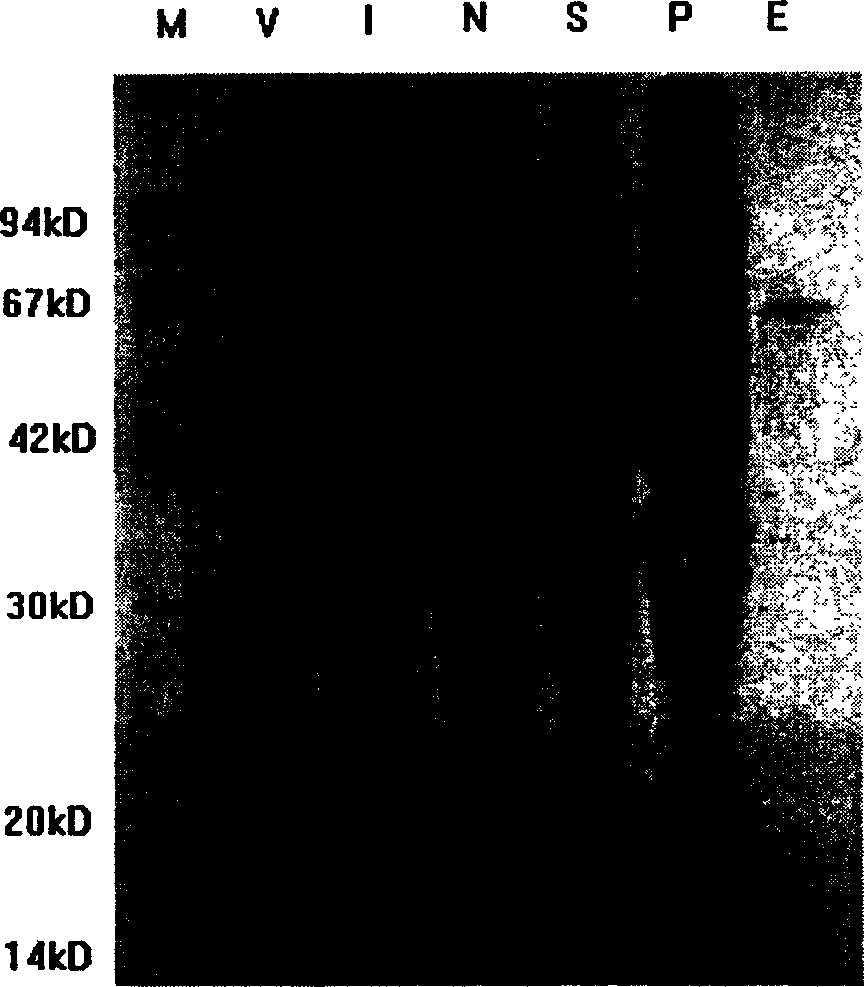
[0037] Example 2 Expression and Purification of Recombinant WP486 Fragment
[0038] The recombinant expression plasmid pGEX-WP486 containing the WP486 gene was transformed into Escherichia coli BL21, and the positive clones were selected and cultured in LB medium containing 100mg / L ampicillin at 37°C until A 600 When = 0.6, add IPTG to a final concentration of 0.1 mmol / L, and collect the bacteria after induction at 37°C for 6 hours. Add ice-cold lysis buffer (1×PBS, 10mM NaHPO 4 , 140mM NaCl, 2.7mM KCl, 1.8mM KH 2 HPO 4 ), ultrasonically lyse the bacteria (300W×10s×10 times), centrifuge at 15,000rpm at 4°C for 20min; mix the supernatant with Glutathione Sepharose 4B equilibrated with lysis buffer in advance, react at 4°C for 10min, and pack the column; use 5-10 Column bed volume of washing buffer (1×PBS) to wash away impurity proteins; elution buffer (50mM Tris-HCl, 10mM reduced glutathione, pH8.0) to elute target protein. The molecular weight of the purified fusion...
Embodiment 3
[0041] Example 3 Antibody Preparation of Recombinant WP486 Fragment
[0042] The purified recombinant protein obtained in Example 2 was emulsified with an equal volume of complete Freund's adjuvant, and the mice were subcutaneously injected with 0.25-0.5 mg / mL emulsified protein, each 0.2 mL. Two weeks later, the same dose of the same antigen emulsified with incomplete Freund's adjuvant was re-injected to boost immunization to produce antibodies, and then boosted immunization every 7 days, at least twice. The titer and specificity of the obtained antisera were analyzed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com