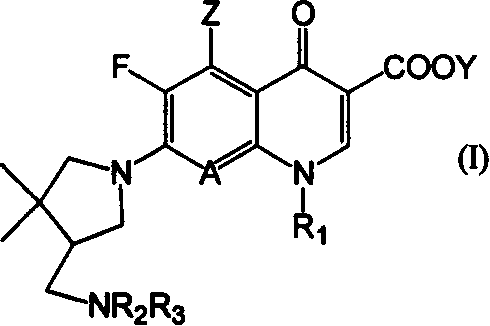
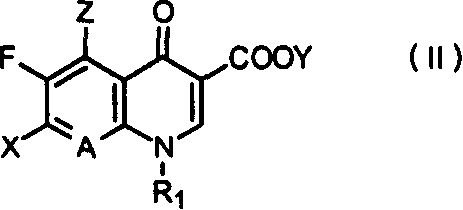
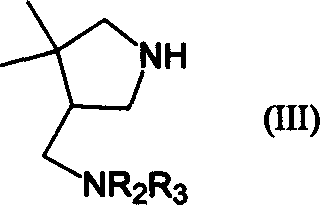
7-(4,4-dimethyl 3-aminomethylpentazane-1-radicle) substituted, quinoline carboxylic acid derivative and its preparation
A dimethyl, carboxylic acid technology, applied in the direction of organic chemistry, antibacterial drugs, etc., can solve the problem of low activity of gram-positive bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 1-benzyl-3,3-dimethyl-2,4-dioxopyrrolidine
[0063] In a 1000ml three-neck flask equipped with a drying tube and a mechanical stirrer, add dry DMF (300ml), ethyl acetoacetate (130g, 1mol), add anhydrous potassium carbonate (286g, 2.2mol) under stirring, and drop Add iodomethane (390 g, 2.8 mol). After completion, the reaction was stirred at room temperature for 7 days. Filter off the solid, and wash the solid with dichloromethane (about 100ml), add water (500ml) to the filtrate, extract with dichloromethane (about 100ml×3), combine the organic layers, wash three times with water, dry over anhydrous magnesium sulfate, filter, and evaporate The solvent was dried to obtain light yellow oil (125 g, yield 79.0%).
[0064] In a 500ml three-necked flask, add the above compound oil (77g, 0.487mol), absolute ethanol (180ml), add bromine (77g, 0.481mol) dropwise under ice bath, complete, stir at room temperature for 2hr, evaporate under reduced pressure Add about hal...
Embodiment 2
[0070] Example 2 1-benzyl-3,3-dimethyl-2-oxo-4-ethoxycarbonylmethylenepyrrolidine
[0071] Add 60% sodium hydride (6.5g) to dry tetrahydrofuran (150ml), stir and cool down to 0°C ~ -5°C, add triethyl phosphonate dropwise, complete, stir at room temperature for 1.5hr, the reaction liquid is jelly-like , compound Example 1 (28.0 g, 0.129 mol) was added dropwise at room temperature. After completion, react at room temperature for 2 hr, dilute with ethyl acetate (300 ml), wash with saturated brine until neutral, dry over anhydrous magnesium sulfate, and evaporate the solvent to The oily product C-7 was obtained by drying (30.95 g, 83.6%).
[0072] 1 HNMR (CDCl 3 )δppm: 1.163~1.276 (3H, t, ethyl ester CH 3 ), 1.316 (6H, s, 3-2×CH 3 ), 4.066~4.135 (2H, q, ethyl ester CH 2 ), 4.342, 4.351 (2H, d, benzyl-CH 2 ), 4.524 (2H, s, 5-CH 2 ), 5.813~5.829 (1H, t, alkene=CH), 7.219~7.346 (5H, m, ArH)
[0073] EIMS: 287(M + ), 258 (M + -CO-H), 91(C 6 h 5 CH 2 + )
Embodiment 3
[0074] Example 3 1-benzyl-3,3-dimethyl-2-oxo-4-ethoxycarbonylmethylpyrrolidine
[0075] Add absolute ethanol (700ml), active nickel and the compound of Compound Example 2 (30.95g, 0.108mol), and react at room temperature under 40-50psi hydrogen pressure for 12hr. TLC shows that the reaction is complete, and the nickel powder is filtered off, and the solvent is evaporated to After drying, a pale yellow oil (29.3 g, 94.0%) was obtained.
[0076] 1 HNMR (CDCl 3 )δppm: 0.970 (3H, s, 3-CH 3 ), 1.187 (3H, s, 3-CH 3 ), 1.187~1.244 (3H, t, ethyl ester CH 3 , J=7.8Hz), 2.197~2.253 (1H, m, 4-CH), 2.403~2.478 (2H, m, side chain CH 2 ), 2.789~2.850, 3.330~3.387 (2H, tt, 5-CH 2 , coupled with hydrocarbons, J=9Hz), 4.060~4.131 (2H, q, ethyl ester CH 2 , J=7.2Hz), 4.297~4.575 (2H, dd, benzyl-CH 2 ), 7.180~7.314 (5H, m, ArH)
[0077] EIMS: 289(M + ), 244 (M + -OEt), 114(C 6 h 5 N=CH 2 ), 91(C 6 h 5 CH 2 + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com