Nickel hydroxide nanotube and its prepn and application
A technology of nickel hydroxide and nanotubes, applied in nickel oxide/nickel hydroxide, nanostructure manufacturing, nanotechnology, etc., to achieve the effect of improving discharge specific capacity and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
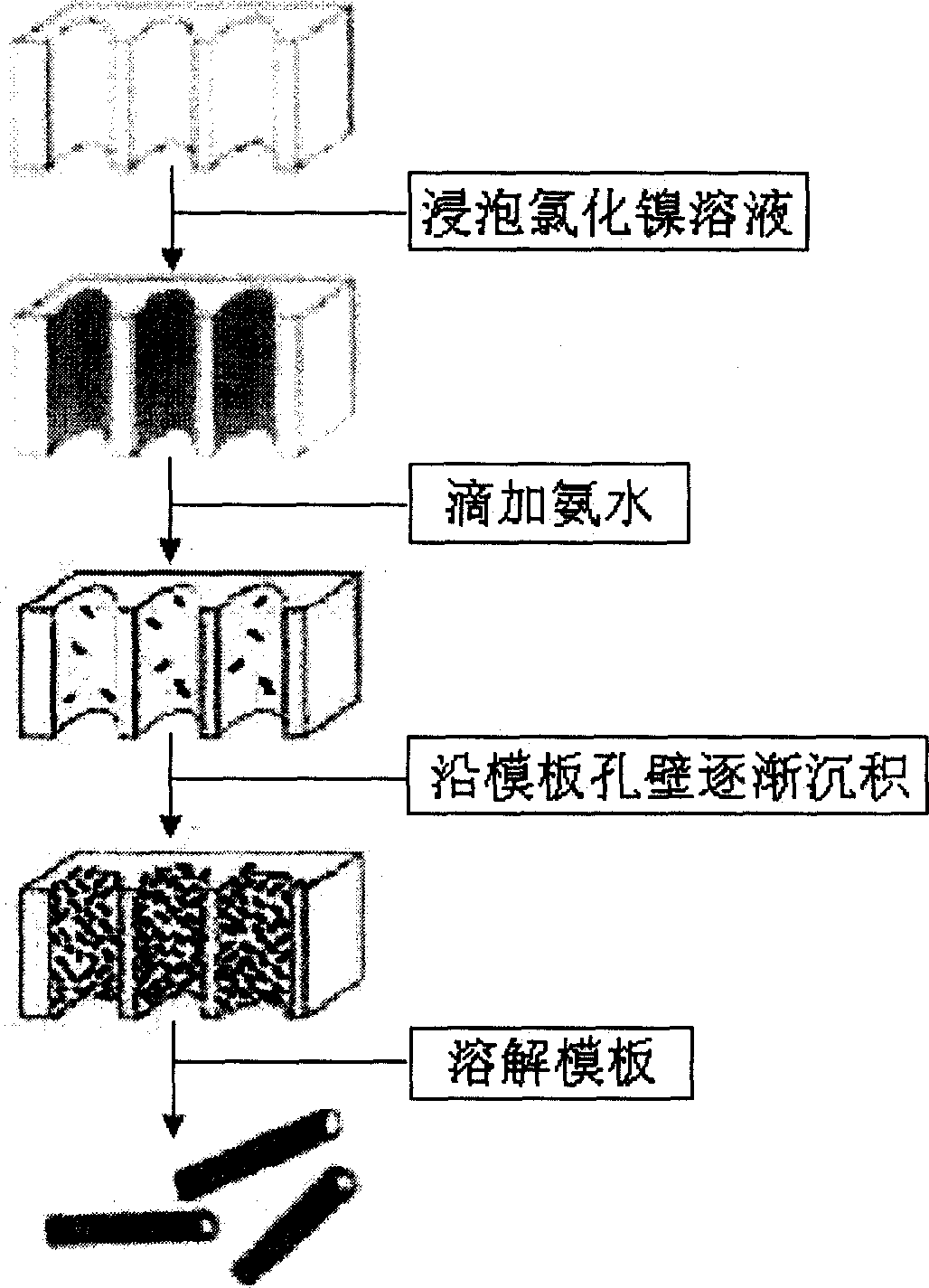
[0027] Embodiment 1: high-purity Ni(OH) 2 Preparation of nanotubes.
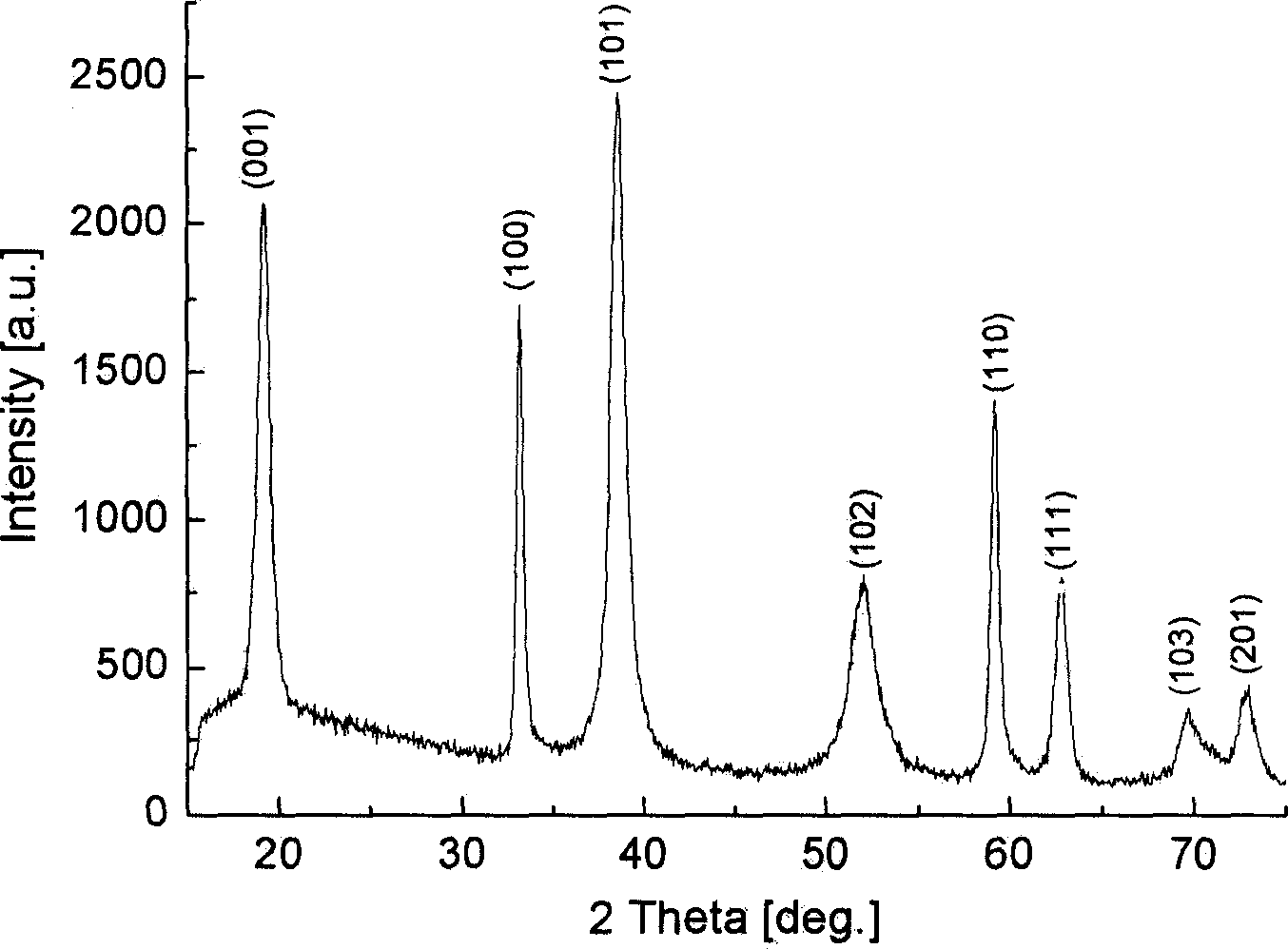
[0028] Experimental steps: (1) put the alumina template (diameter 47mm, pore size 200nm, thickness 60μm, Whatman, England) into 0.4M NiCl 2 Soak in the solution for half an hour to make the NiCl 2 The solution is fully immersed in the micropores of the template; (2) from NiCl 2 Remove the template from the solution and dry it. Slowly add ammonia water with a concentration of 1M to the surface of the template. Under the action of gravity and capillary action, the ammonia water penetrates into the micropores and penetrates the template, and the NiCl attached to the wall of the template hole 2 The reaction produces Ni(OH) 2 . Under visible light, the color of the template can be observed by the human eye gradually changing from white to light green. (3) After repeating steps (1) and (2) 4 times, rinse the template with distilled water, and dissolve the template in 2M NaOH solution. (4) Collect the green ...
Embodiment 2
[0033] Nanotube Ni(OH) prepared according to embodiment 1 2 Or commercial spherical nickel hydroxide (Tanaka Chemical, Japan) is used as the positive electrode active material, carbon black is used as the conductive agent, polytetrafluoroethylene (PTFE) is used as the binder, and nickel foam is used as the current collector to make the positive electrode sheet. Active materialNi(OH) 2 , carbon black, PTFE mass percentage is Ni(OH) 2 : Carbon black: PTFE = 85:10:5. Accurately weigh each component, grind it fully and mix it uniformly, make the mixture into a paste with an appropriate amount of absolute ethanol, and apply it evenly on the nickel foam substrate, dry it at 80°C for 1 hour, and use a press The tablet machine presses the positive electrode film with a thickness of about 0.4mm as the positive electrode sheet. Use hydrogen storage alloy powder with a discharge capacity exceeding 200% of the positive electrode capacity (mixed rare earth hydrogen storage alloy MmNi pr...
Embodiment 3
[0035] Nanotube Ni(OH) prepared according to embodiment 2 2 Electrode and spherical Ni(OH) 2 The electrodes were subjected to discharge tests at different temperatures (20, 40, 60°C) and different current densities (50, 100, 150mA / g). Figure 6 Nanotube Ni(OH) 2 Electrodes and spherical Ni(OH) 2 Comparison of discharge specific capacity of electrodes at different temperatures and different current densities. Figure 6 It reflects the discharge specific capacity performance of the two electrodes at different temperatures and different current densities. It can be seen from the figure that the discharge specific capacity of the two electrodes decreases with the increase of temperature and current density, but at the same temperature and current density, the nanotube Ni(OH) 2 The discharge specific capacity of the electrode is always higher than that of spherical Ni(OH) 2 The specific discharge capacity of the electrode. For example, when discharged at 20°C and a current d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com