Process for recovering strontium and aluminium from metal strontium residue
A metal strontium and residue technology, applied in the field of inorganic chemical industry, can solve the problems of high production cost and poor product purity, and achieve the effects of energy saving, low price and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
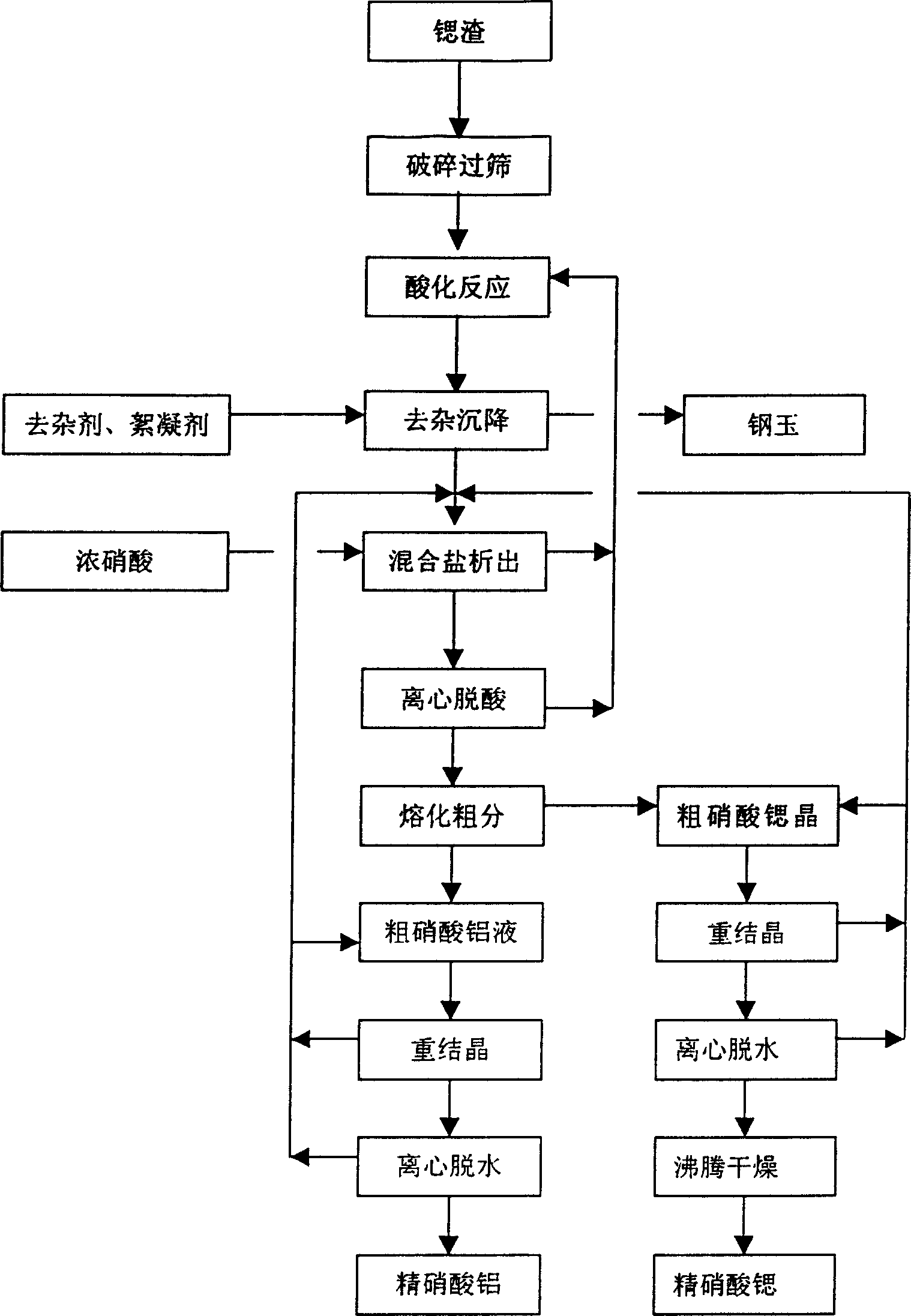
[0028] Such as figure 1 or figure 2 The process flow shown: Use dry residue powder below 20 mesh to react with 20%-60% nitric acid, remove barium with 5%-30% sulfuric acid, then use 10-500ppm polyacrylamide aqueous solution to flocculate and settle, and discharge the sediment. ①In the preparation of nitrate, use 90%--98% concentrated nitric acid in the supernatant to precipitate mixed salts, and the concentration of nitric acid in the precipitated mother liquor is within 30%--60%. The dilute nitric acid solution is returned to use, and after the mixed salts are centrifugally deacidified, Heat to 85--100°C to melt the aluminum nitrate into a liquid, heat preservation and sedimentation to separate crude strontium nitrate crystals and crude aluminum nitrate molten liquid, then recrystallize the crude salt to separate strontium nitrate and aluminum nitrate crystals, and return the mother liquor for use. Strontium nitrate is centrifuged and dehydrated to obtain the product, and a...
Embodiment 2
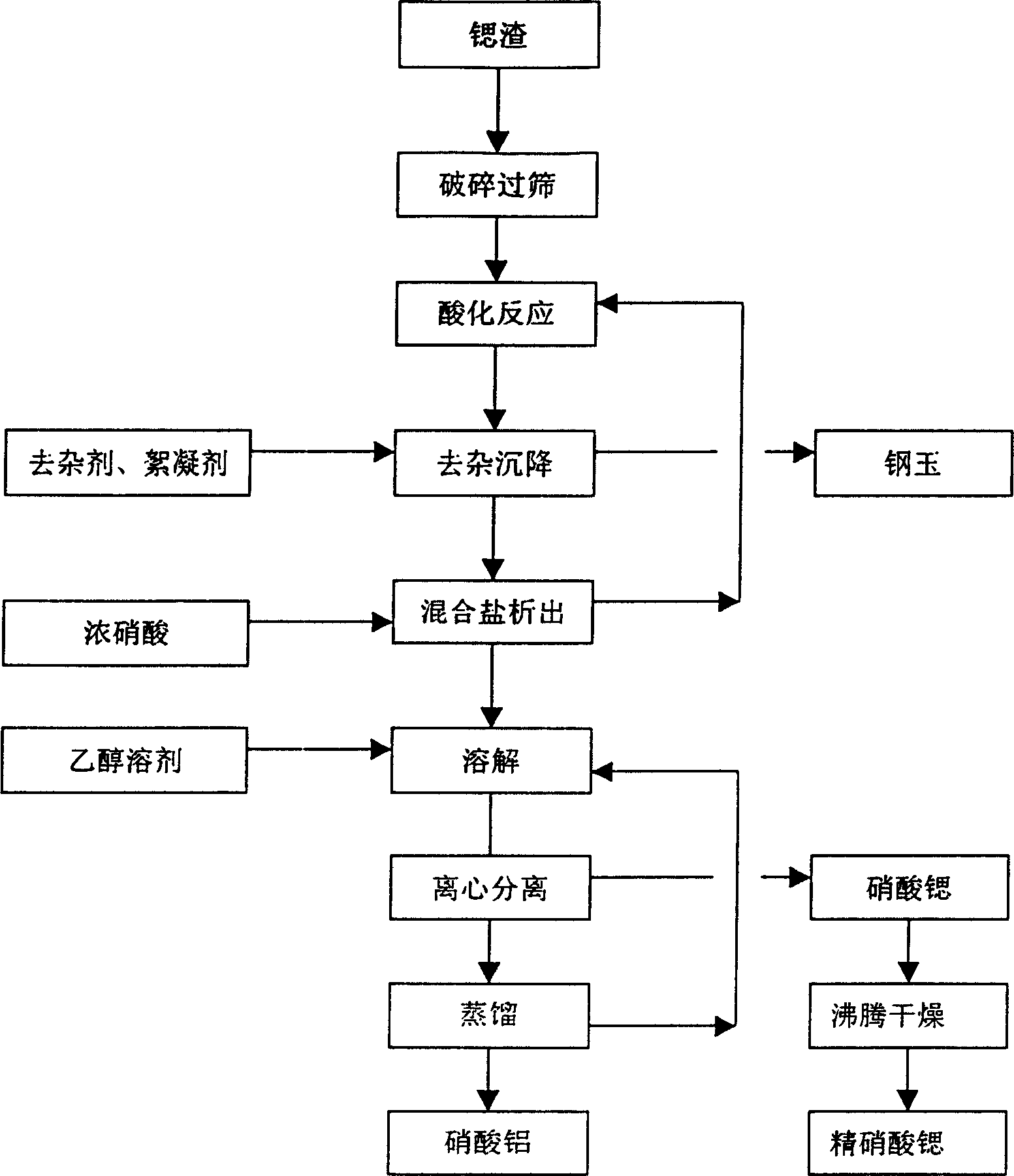
[0030]The difference between this embodiment and embodiment 1 is that the present embodiment uses 15%--36% hydrochloric acid. Wherein the supernatant liquid in the preparation of the chloride salt is concentrated and evaporated to obtain the refined salt by recrystallizing the ternary phase diagram of strontium chloride-aluminum chloride-water, or directly concentrated and evaporated to crystallize the strontium chloride-aluminum chloride mixed crystal, The mixed salt is dissolved with more than 95% ethanol to separate the insoluble strontium chloride, the solution is distilled, the temperature is lower than 90°C, the liquid is discharged from the kettle, cooled and crystallized to obtain aluminum chloride hexahydrate, and the mother liquor is returned for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com