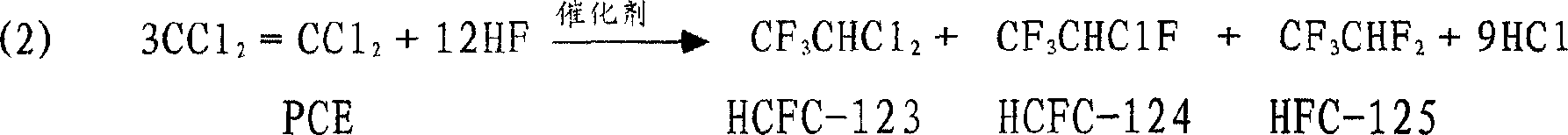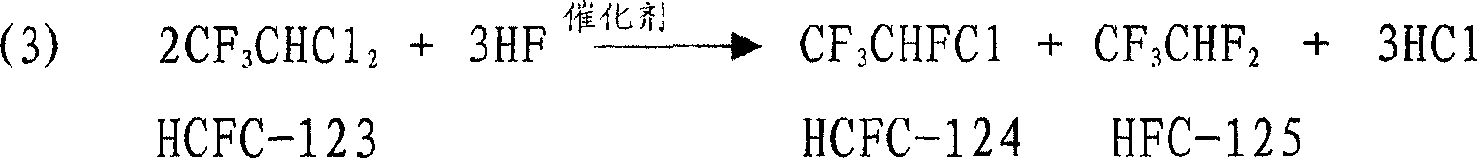Florination catalyst, its manufacturing method and use
A fluorination catalyst, catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. The effect of good selectivity, simple method and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve trivalent chromium soluble salts (chromium chloride, chromium nitrate, chromium sulfate) in water, react with precipitant ammonia water at 60°C, adjust the pH value of the reaction solution to be in the range of 7.5 to 8.5, and make it under the condition of stirring The resulting slurry was filtered, washed with deionized water until neutral, and then dried at 120°C for 12 hours. Produce Cr(OH) 3 . The obtained Cr(OH) 3 The weight ratio with Mg powder, Zn powder, Ni powder and graphite powder is 55:30:5:5:5, mixed evenly, and pressed into tablets. In the tubular reactor, 100~400℃N 2 Roast in the atmosphere for 6 hours, and then feed a mixture of nitrogen and hydrogen fluoride at a ratio of 4:1, fluoride at 120°C for 4 hours, then increase the temperature to 350°C at a rate of 1°C / min, and continue fluorinating for 8 hours to obtain active Fluorination catalyst.
[0029] The pore distribution of the catalyst was measured by the BET low-temperature nitrogen a...
Embodiment 2
[0063] The catalyst preparation process is substantially the same as in Example 1, except that Cr(OH) 3 The weight ratio of Mg powder, Zn powder, Ni powder and graphite powder is 70:15:5:5:5.
[0064] The pore distribution of the catalyst was measured by the BET low-temperature nitrogen adsorption method, and the micropore ratio of the fluorinated catalyst was measured to be 30%.
[0065] 50ml of the fluorination catalyst prepared above was used in the reaction of the fluorine-chlorine exchange reaction to synthesize a series of HFCs in Example 1. The reaction product was washed with water and alkali, and analyzed by gas chromatography after removing HCl and HF. The results are shown in Table 1.
[0066] Table 2
[0067] Material ratio / molar ratio
[0068] Reaction Temperature / °C Contact Time / s Conversion / % Selectivity / %
[0069] (HF / halogenated hydrocarbon)
[0070] 1 260 8 / 1 3 81 91
[0071] 1 280 8...
Embodiment 3
[0085] The catalyst preparation process is substantially the same as in Example 1, except that Cr(OH) 3 The weight ratio of Mg powder, Zn powder, Ni powder and graphite powder is 80:5:5:5:5.
[0086] The pore distribution of the catalyst was measured by the BET low-temperature nitrogen adsorption method, and the micropore ratio of the catalyst was measured to be 30%.
[0087] 50ml of the fluorination catalyst prepared above was used in the reaction of the series of HFCs synthesized by the fluorine-chlorine exchange reaction in Example 1. The reaction product was washed with water and alkali, and analyzed by gas chromatography after removing HCl and HF. The results are shown in Table 3.
[0088] table 3
[0089] Material ratio / molar ratio
[0090] Reaction Temperature / °C Contact Time / s Conversion / % Selectivity / %
[0091] (HF / organic)
[0092] 1 260 8 / 1 3 77 90
[0093] 1 280 8 / 1 3 85 85
[0094] 2 280 8 / 1 20 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com