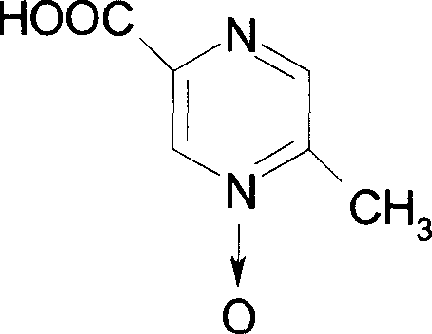
Preparation method of acymose
A technology for intermediates and reaction kettles is applied in the field of preparation of the compound axilimus, and can solve the problems of high production cost, difficult industrialized production, low total yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
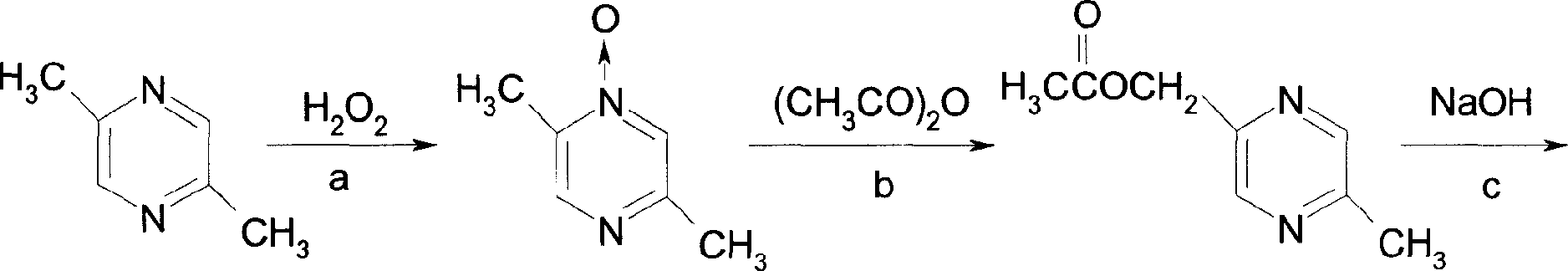
[0020] Embodiment 1: the preparation of intermediate I
[0021] 200g (1.85mol) of 2,5-dimethylpyrazine, 200ml of 30% hydrogen peroxide, and 900ml of acetic acid were stirred and reacted at 70°C for 12 hours, the solvent was evaporated under reduced pressure, 500ml of ice water was added to the residue, and 20% sodium hydroxide solution was used to Adjust the pH to >10, extract with chloroform, and evaporate the solvent after drying. The residue is recrystallized with toluene, filtered with suction, and dried to obtain 177 g of a white solid with a yield of 77%, m.p.105-107°C.
Embodiment 2
[0022] Embodiment 2: the preparation of intermediate II
[0023] 270ml of acetic anhydride, 78g of sodium acetate and 1750ml of glacial acetic acid were added to the reaction kettle, and after stirring for 10 minutes, if the intermediate I was 133g (1.07mol), after reflux reaction for 5 hours, the solvent was first evaporated under reduced pressure, and then the fractions were collected. 151g of intermediate II was obtained with a yield of 85%, b.p.120-123°C / 12mmHg.
Embodiment 3
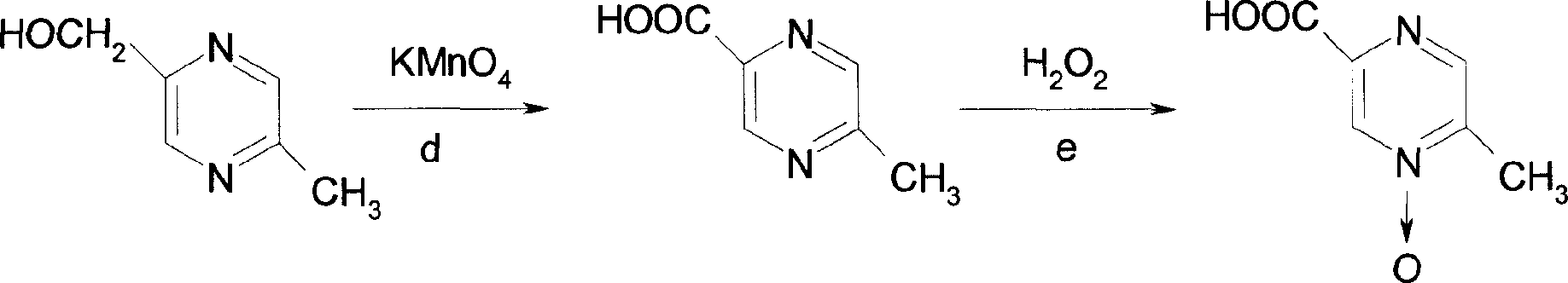
[0024] Embodiment 3: the preparation of intermediate III
[0025] Intermediate II 64g (0.38mol) and 300ml of 10% sodium hydroxide solution were stirred at room temperature for 24 hours, extracted with ethyl acetate, anhydrous MgSO 4 After drying, it was concentrated to obtain 39g of intermediate III with a yield of 83%, m.p.34-36°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com