High efficiency production method for recombinant silkworm antibacterial peptide CM4
A silkworm antimicrobial peptide and production method technology, applied in the field of genetic engineering, to achieve high expression efficiency, simple separation and purification, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
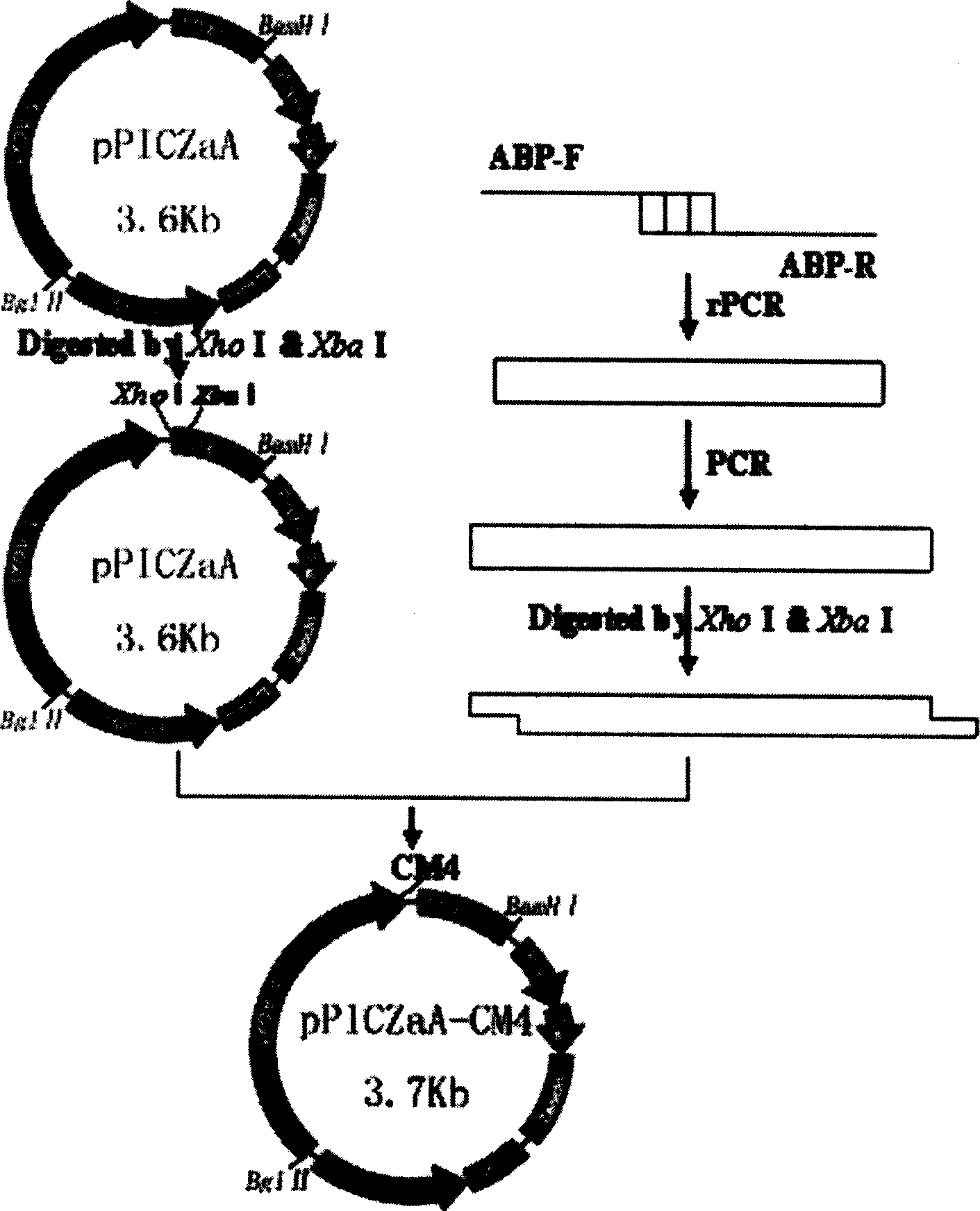
[0028] (1) Construction of expression vector: According to the preferred codons of Pichia pastoris gene translation and the amino acid sequence of antimicrobial peptide CM4, and the multiple cloning site of pPIC9, two primers were designed and synthesized as follows:
[0029] ABP-F 5′agatggaagattttcaagaagatcgagaaggtcggtcaaaacatcagagacggtatcgt3′
[0030] ABP-R 5′aatagtagcagcttgaccgacgacagcgacagctggaccagccttgacgataccgtctc3′
[0031] The enzyme cutting sites are Xho I and Xba I, and the expression plasmid is pPICZaA. For vector construction, see figure 1 ;
[0032] (2) Construct genetically engineered bacteria: transform the host bacteria with the above-mentioned expression vector, and the host bacteria strain is yeast GS115;
[0033] (3) Induced expression: Use BMGY at 28°C to 30°C to ferment and cultivate the expression engineered bacteria for about 20 hours until the OD600 reaches 2-6; centrifuge, resuspend with BMMY until the OD600 is about 1.0, culture at 20°C to 30°C, eve...
Embodiment 2
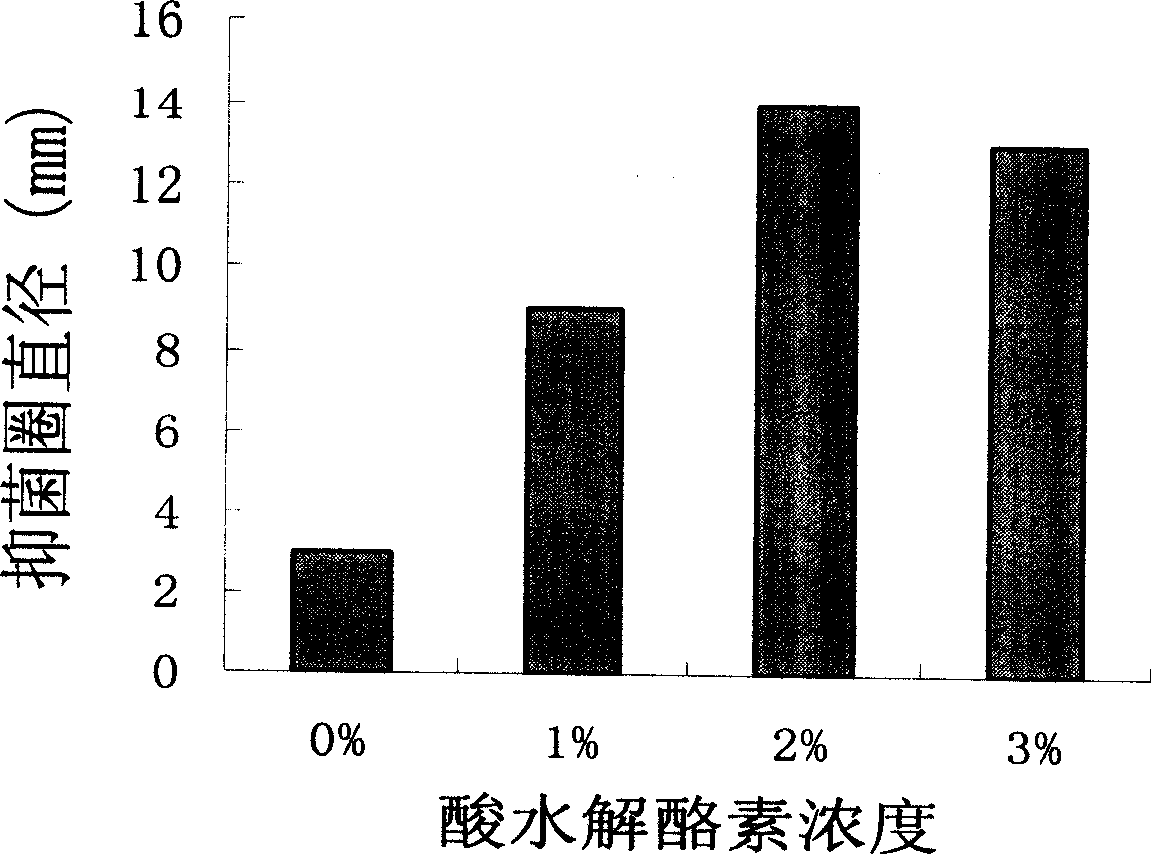
[0036] Example 2: basically the same as Example 1, the difference is that during the process of inducing expression, 1-3% (W / V) acid hydrolyzed casein is added to BMMY to inhibit degradation. Analysis of the relationship between the amount of acid hydrolyzed casein and the expression product is as follows: figure 2 shown.
[0037] Adopt molecular sieve chromatography (separation medium is sephadex G50), equilibrate 5 column volumes (1.5×40cm) with 0.05M, pH5.1 ammonium acetate buffer solution, flow rate is 0.5ml / min; Above-mentioned sample loading; 0.05M, pH5 .1 Ammonium acetate buffer elution (0.15ml / min), chromatography and antibacterial activity analysis Figure 5 As shown, the eluate of antibacterially active protein was collected.
Embodiment 3
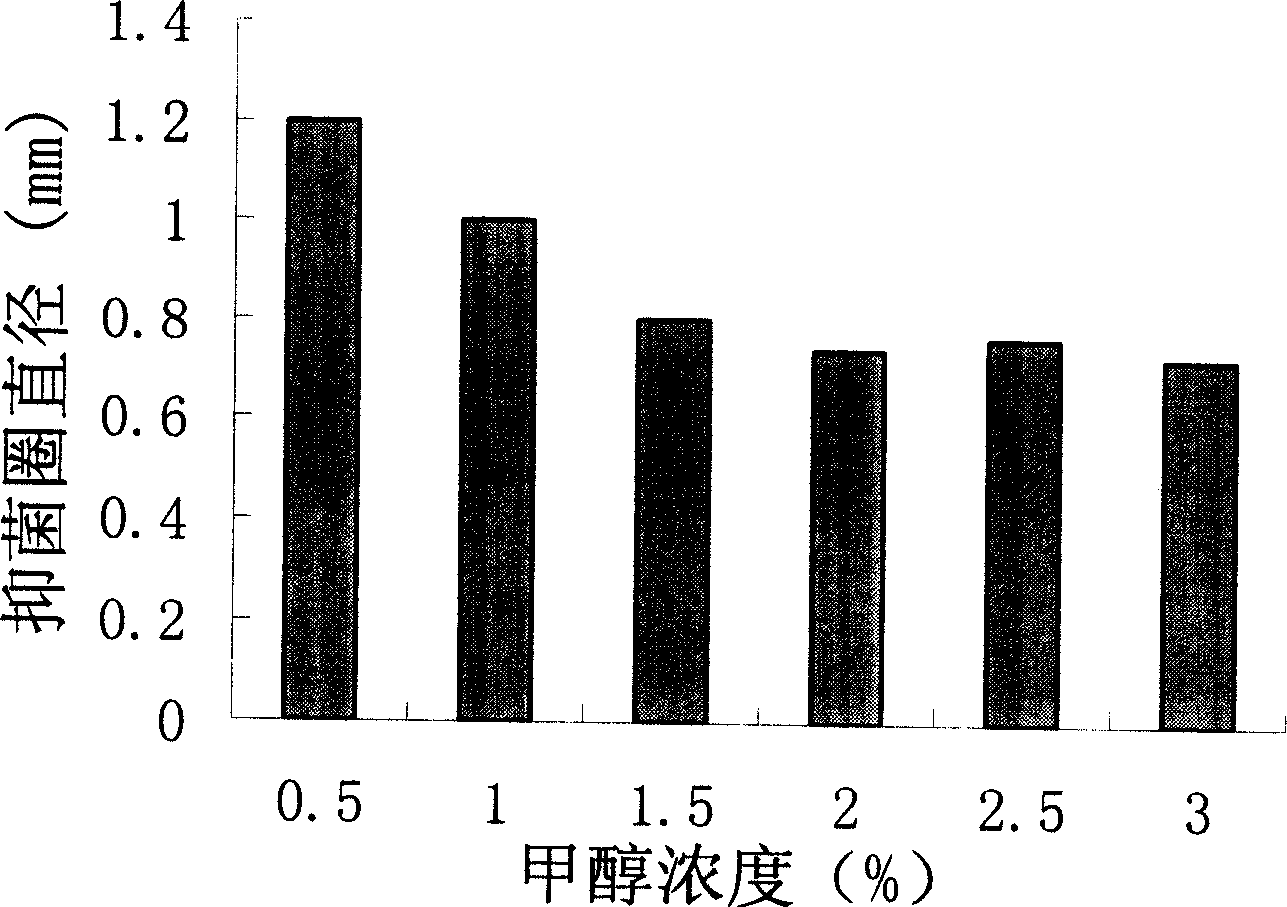
[0038] Embodiment 3: the same method as Example 2, analyze the relationship between methanol induction culture time and expression product as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com