Polmerization method of catalysis chain transfer for methyl methacrylate
A technology of methyl methacrylate and catalytic chain transfer, which is applied in the field of polymerization of organic substances, can solve the problem of not using microwave technology, etc., and achieves the effects of increasing chain transfer constant, accelerating reaction speed, and improving reaction speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
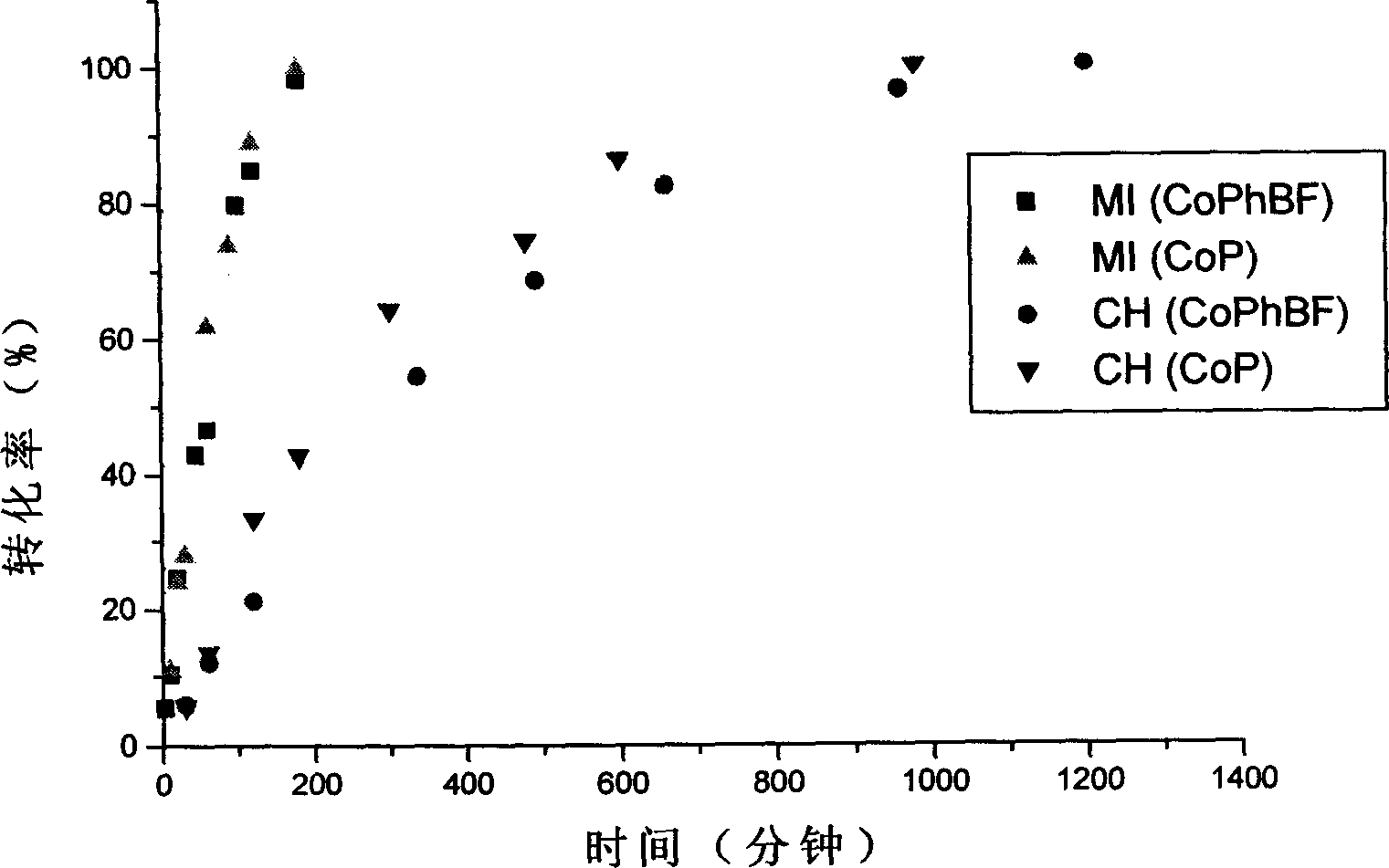
[0017] Embodiment 1: Catalyzed chain transfer polymerization of methyl methacrylate with 5,10,15,20-tetraphenyl-21H, 23H-cobaltpurine (CoP) as a catalytic chain transfer reagent under microwave irradiation:
[0018] Monomer methyl methacrylate (methyl methacrylate, MMA, Shanghai Reagent Company) was washed with deionized water to neutrality after being washed with alkali to colorless, and then used by vacuum distillation after drying with anhydrous magnesium sulfate; the initiator azobis Isobutyronitrile (AIBN, Shanghai Reagent Company) was recrystallized twice with ethanol and then dried in vacuum at room temperature; the catalytic chain transfer reagent CoP was purchased directly from Aldrich; the rest of the reagents were products of Shanghai Reagent Factory, and were used after being treated by the usual method.
[0019] The polymerization device adopts the single-mode microwave reactor of the Discover series of the American CEM company. The microwave radiation power is adj...
Embodiment 2
[0025] Embodiment two: Catalyzed chain transfer polymerization of methyl methacrylate with bis(boron trifluoride) bis(diphenyl)cobaloxime (CoPhBF) as catalytic chain transfer reagent under microwave irradiation
[0026] Monomer methyl methacrylate (Shanghai Reagent Company) was purified by vacuum distillation after alkali washing, water washing and drying. The initiator azobisisobutyronitrile (AIBN) was purified by recrystallization from ethanol. Phenylcobaloxime was synthesized according to literature method (Andreja BakaE, Mark E. Brynildson, and James H. Espenson, Inorganic Chemistry, 1986, 25, 4108-4114).
[0027] Methyl methacrylate and toluene were sparged with argon for three hours and then transferred to an anaerobic operating box. Add 50ml toluene to 2.8mg CoPhBF to make a catalyst solution. 60 mg of AIBN was dissolved in a mixed solution of 12 ml of methyl methacrylate and 18 ml of toluene to prepare an initiator solution. Add 5ml initiator solution in each reacti...
Embodiment 3
[0032] Embodiment three: Catalytic chain transfer polymerization of methyl methacrylate with bis(boron trifluoride) bis(dimethyl)cobaloxime (CoBF) as catalytic chain transfer reagent under microwave irradiation
[0033] Monomer methyl methacrylate (Shanghai Reagent Company) was purified by vacuum distillation after alkali washing, water washing and drying. The initiator azobisisobutyronitrile (AIBN) was purified by recrystallization from ethanol. Methylcobaloxime was synthesized according to literature method (Andreja BakaE, Mark E. Brynildson, and James H. Espenson, Inorganic Chemistry, 1986, 25, 4108-4114).
[0034] Methyl methacrylate and toluene were sparged with argon for three hours and then transferred to an anaerobic operating box. Add 50ml of toluene to 2.8mg of CoBF to make a catalyst solution. 60 mg of AIBN was dissolved in a mixed solution of 12 ml of methyl methacrylate and 18 ml of toluene to prepare an initiator solution. Add 5ml of initiator solution to each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com