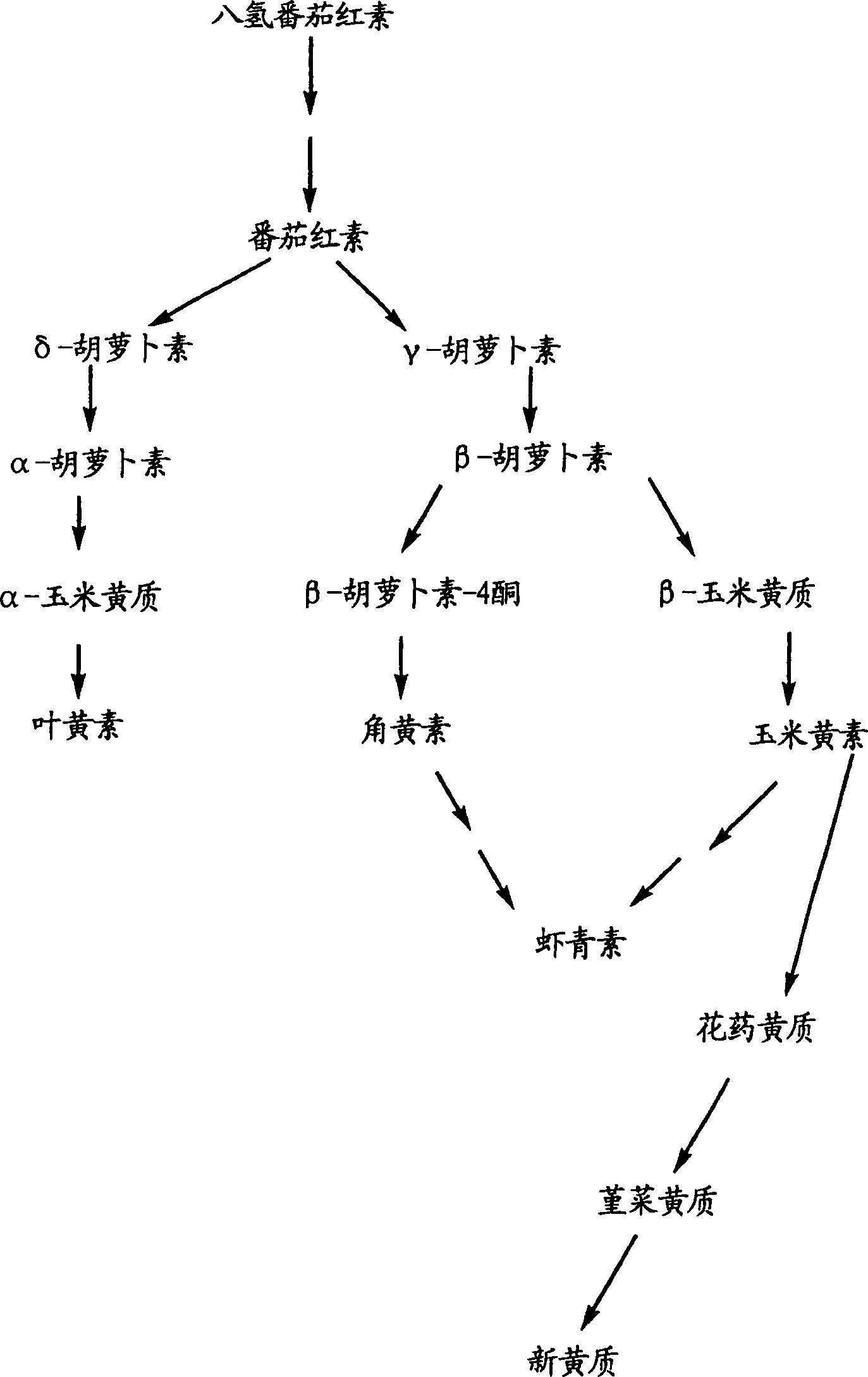
4-ketocarotenoids in flower petals
A carotene and petal technology, applied in angiosperms/flowering plants, biochemical equipment and methods, fermentation, etc., can solve the problems of not giving carotenoid yield, not giving total carotenoids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] Embodiment 1: Construction of β-carotene ketolase (β-carotene oxygenase) expression vector
[0265] A. β-carotene ketolase from Haematococcus pluvialis
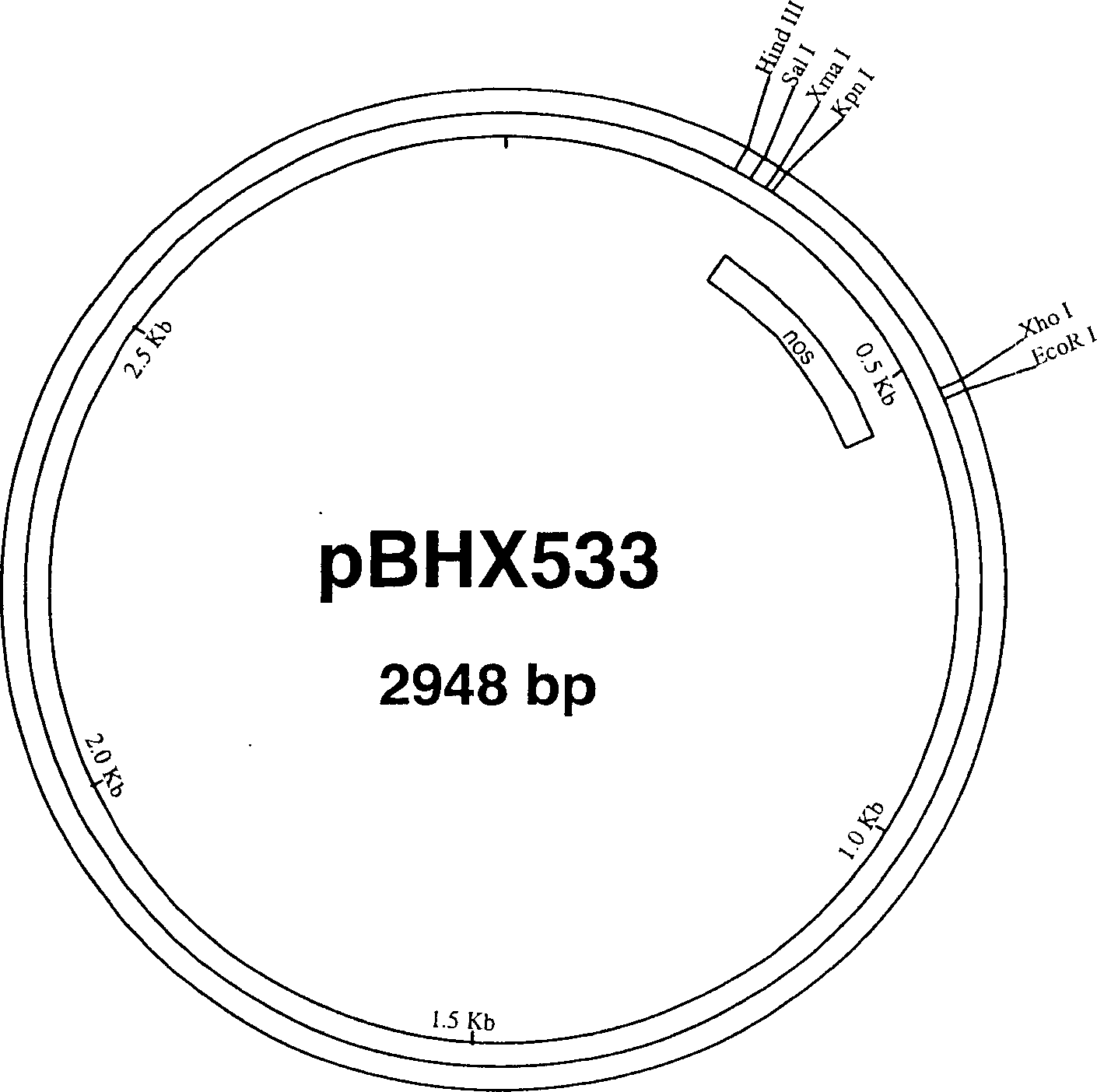
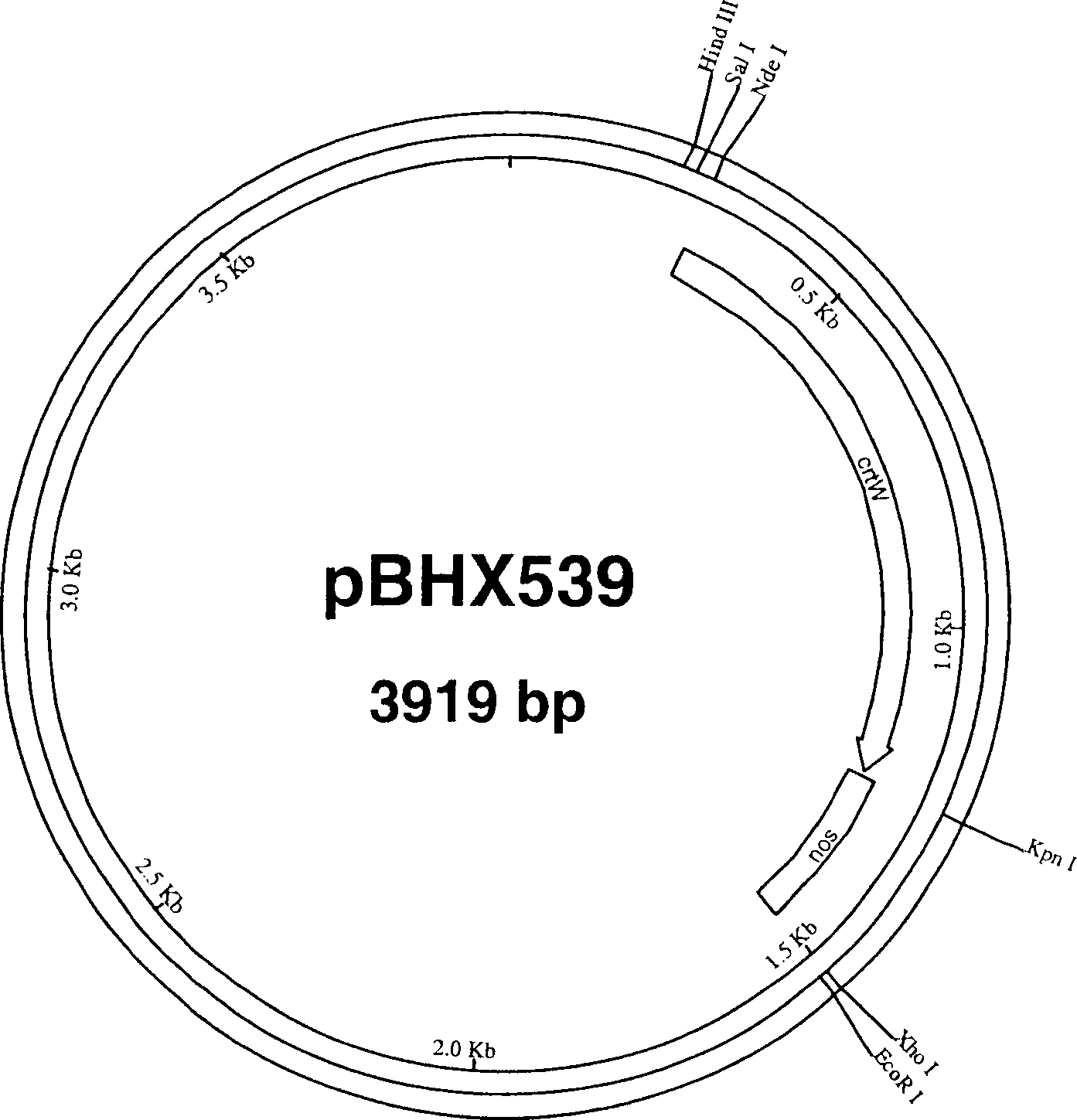
[0266] Utilizing the gene clone obtained by Dr. Toshihiro Toguri, Kirin Brewery Co., Ltd as a template, the β-carotene ketolase gene (crtW) from Haematococcus pluvialis was prepared by polymerase chain reaction (PCR) [see Kajiwara et al., Plant Mol Biol., 29(2):343-352 (1995)]. Use specific primers to introduce a Kpn I restriction enzyme site (crtW-L28: GCCAGTGCCAA GGTACCCTTGTCATGCC; SEQ ID NO: 1) at the 3' end of the gene, and an Nde I site (crtW-U28: CCGGGGATCCTCTACATATGCACGTCGC; SEQ ID NO: 1) at the 5' end. ID NO: 2). After digestion with KpnI and NdeI, the crtW gene was ligated into the plasmid pBHX533 containing the nopaline synthase polyadenylation signal ( figure 2 )middle. The resulting vector was named pBHX539 ( image 3 ).
[0267] Sources were prepared by PCR using primers introducing an Xma I sit...
Embodiment 2
[0286] Example 2: Binary Vectors
[0287] Plasmid pBHX103
[0288] The plasmid containing the 5' flanking region of the fairy fan LIS1 gene was obtained from Dr. Eran Pichersky, University of Michigan. An approximately 1 kb fragment containing the LIS1 5' flanking region was synthesized by PCR using primers: BHX30: CCAAGCTTATCTAATAATGTATCAAAATC (SEQ ID NO: 11) and BHX36: CAGCCC GGGATGGTTGTCTTGTTTAAGGTGG (SEQ ID NO: 12). These primers were designed to anneal to the 5' flanking region at one end and the 5' untranslated leader at the 3' end. The PCR product was digested with restriction enzymes Hind III and Sma I that cut at the 5' and 3' ends of the fragment, respectively. The digested fragment was gel purified and subsequently inserted into a Hind III and Sma I digested plasmid containing the multiple cloning site region (MCS) followed by the nos polyA signal region to generate the LIS1::MCS::nos transgene (Designated as plasmid pBHX103).
[0289] Plasmid pBHX107
[0290...
Embodiment 3
[0328] Example 3: EMS Treatment of Marigold 'Scarletade'
[0329] Marigold carotene marigold (from PanAmerican Seed Co. 622 Town Road, West Chicago) designated 'Scarletade' was treated with ethyl methanesulfonate (EMS, commercially available from Sigma Chemical Co., St. Louis, MO 63178). , IL 60185 commercially available) seeds. Approximately 2,500 seeds were added to 400 ml of 0.4% (v / v) or 0.8% (v / v) EMS and stirred gently at room temperature for 8 hours. During the 4 hours after EMS treatment, the seeds were washed 16 times with 400 ml of distilled water each time with continuous agitation. Then the processed seed is defined as M 1 Seeds, sown in trays containing soilless ceramic mix.
[0330] After a few weeks, the seedlings are transplanted into pots containing a soilless ceramic mix and grown in a greenhouse. The flowers produced by these plants are naturally self-pollinating. The resulting seeds were harvested from approximately 2,300 plants, defined as M 2 seed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com