Technique for preparing pharmaceutical product of Breviscapine
A technology for preparing scutellarin and its preparation technology, which is applied in the field of preparation technology of raw materials, can solve the problems of improving the purity of scutellarin and eliminating side effects that have not been reported, and achieves easy industrial production, solving instability, and process Effects in simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
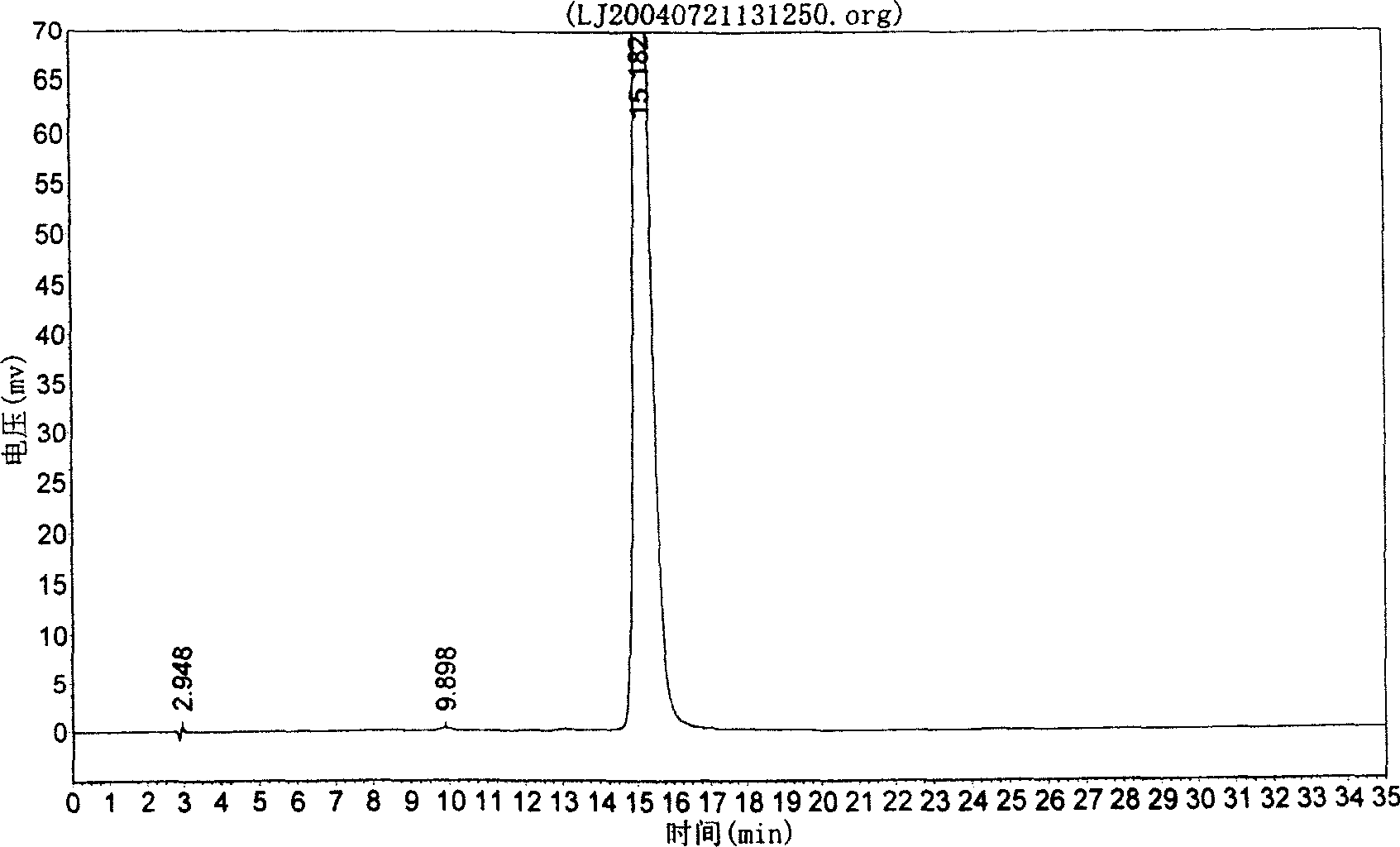
[0021] Weigh 1000g of commercially available scutellarin crude drug, add 5 times the weight of water, adjust the pH value to 7 with 25% sodium metaphosphate test solution, dissolve completely, filter, add 8 times of acetone to the filtrate to precipitate, stir while adding, Make the precipitation complete, stand still for 10 hours, filter, add acetone to wash three times, then move the precipitate to another container, add 5 times the amount of 30% acetone, stir well, add 20% hydrochloric acid to adjust the pH to 1-2 and let stand After 10 hours, filter with suction, wash with water until neutral, wash once with ethanol, and dry to obtain refined scutellarin. The content of scutellarin by HPLC analysis is 99.5315%, as shown in the attached drawing.
[0022] Detection method: chromatographic conditions and system suitability test (using octadecyl bonded silica gel as filler: methanol-0.1% phosphoric acid solution (40:60) as mobile phase; detection wavelength is 335nm. The number...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com