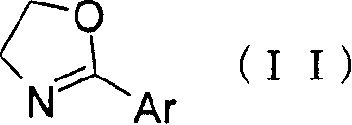Novel benzamide derivatives and process for production thereof
A technology of benzamide and derivatives, applied in the new field of benzamide, can solve the problems of low efficiency, not an industrial manufacturing method, difficulty in estimating and controlling reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
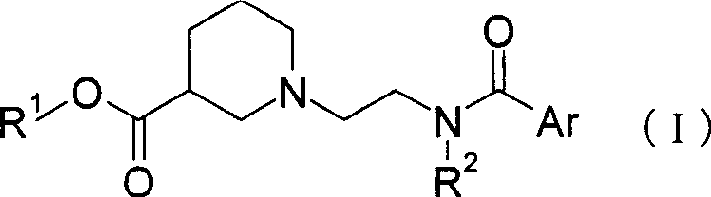
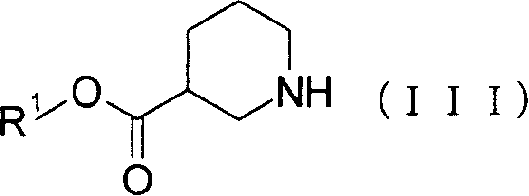
[0120] To 120.0 g of the compound of Reference Example 11 were added 1,200 ml of toluene and 137.07 g of ethyl (R)-piperidine-3-carboxylate. (R)-Piperidine-3-carboxylic acid ethyl ester was washed with 600 ml of toluene. Thereafter, 145.1 g of p-toluenesulfonic acid monohydrate was added thereto, followed by washing with 600 ml of toluene. The reaction mixture was heated and the solvent was distilled under normal pressure to evaporate 600 ml therefrom. Afterwards, the reaction mixture was stirred at reflux for 27 hours. After the mixed solution was cooled, 960 ml of EtOAc and 960 ml of 4% (w / v) NaHCO were added 3 aqueous solution. The reaction mixture was allowed to stand at about 35°C to separate the layers. The obtained organic layer was washed with 960 ml of 4% (w / v) NaHCO 3 Wash twice with aqueous solution. The organic layer was concentrated under vacuum to give ethyl (R)-1-{2-[(4-fluorobenzoyl)amino]ethyl}piperidine-3-carboxylate with a purity of 82.8%.
[0121] NM...
Embodiment 2
[0124] To 230 g of the compound of Example 1 was added 690 ml of EtOH, and then 345 ml of water was added thereto. After cooling, an aqueous NaOH solution (42.8 g of NaOH / 480 ml of water) was added, followed by stirring at 25° C. or lower for 2 hours. After cooling, concentrated hydrochloric acid was added to the reaction mixture to adjust the pH to 3.0. The solution was concentrated under vacuum, 1,000 mL of toluene was added to the residue, and the mixture was concentrated under vacuum to give (R)-1-{2-[(4-fluorobenzoyl)amino]ethyl}piperidine -3-Carboxylic acid with a purity of 86.3%.
[0125] NMR (90°C): δ1.46-1.60 (1H, m), 1.79-2.05 (3H, m), 2.75-3.60 (7H, m), 3.68 (2H, q), 7.20-7.27 (2H, m) , 7.95-8.03 (2H, m), 8.74 (1H, brs).
[0126] FAB-MS m / z: 295 (M + +1).
Embodiment 3
[0128] 810 ml of DMF and 120.8 g of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline monohydrochloride were added to 206.4 g of the compound of Example 2, followed by stirring and cooling. After that, 53.22 g of triethylamine was added at 12° C. or lower, and then 217 ml of DMF was added. Then 21.32 g of HOBt was added at 5°C or lower, followed by 121.0 g of WSC.HCl at 5°C or lower. The reaction mixture was stirred at 0-4°C for 15.5 hours. 340 mL of water, 2,000 mL of EtOAc and 550 mL of 8% (w / v) NaOH aqueous solution were added to the reaction mixture, and then the layers were separated. EtOAc (1,000 mL) was added to the aqueous layer and the layers were separated. After that, the organic layers were mixed and washed twice with 700 mL 8% (w / v) NaOH aqueous solution and 300 mL water. After the organic layer was washed with 900 ml of water, it was concentrated under vacuum to give (-)-N-{2-[(R)-3-(6,7-dimethoxy-1,2,3,4-tetra Hydroisoquinoline-2-carbonyl)piperidino]ethyl}-4-fluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com