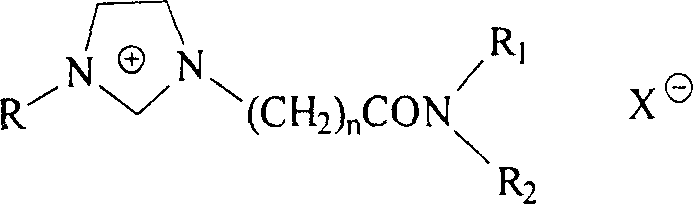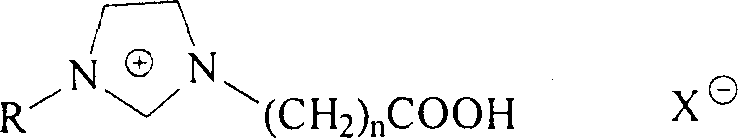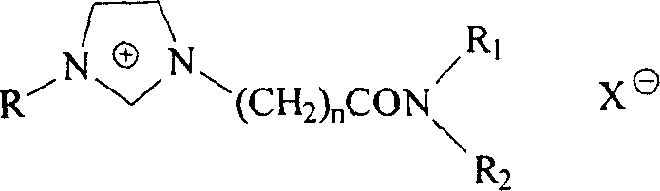Imidazole type functional ion liquid having N-substituted amide structure fragment, and its prepn. method
A technology of ionic liquids and structural fragments, applied in the direction of organic chemistry, etc., to achieve good application prospects, improve efficiency, improve catalytic activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. Preparation of 1-ethyl-3-(N,N-diethylcarbamoylmethyl) imidazole tetrafluoroborate (DEEImBF 4 )
[0046] A, prepare 1-ethyl-3-carboxymethylimidazolium tetrafluoroborate (CMEImBF 4 ) ionic liquid
[0047] Chloroacetic acid 94g, (1.0mol), was added in batches to N-ethylimidazole 94g, (1.0mol), heated at 70-80°C for 4h, cooled, washed twice with 20mL acetonitrile, and evaporated to dryness to obtain 1- Ethyl-3-carboxymethylimidazolium chloride (CMEImCl), yield 95%. CMEImCl94g, (0.5mol) and NaBF 4 54g, (0.5mol) was dissolved in 500mL water, reacted at 80°C for 4h, cooled slightly, fully extracted with dichloromethane, combined organic phase, MgSO 4 Drying, evaporation of solvent to obtain viscous liquid 1-ethyl-3-carboxymethylimidazolium tetrafluoroborate (CMEImBF 4 ), yield 86%.
[0048] B. Preparation of 1-ethyl-3-(N, N-diethylcarbamoylmethyl) imidazole tetrafluoroborate (DEEImBF 4 ) ionic liquid
[0049] CMEImBF 4 Add 0.2 (mol), diethylamine (0.2mol), ...
Embodiment 2
[0051] Example 2. Preparation of 1-ethyl-3-(N-n-butylcarbamoylmethyl) imidazole tetrafluoroborate (BEImBF 4 )
[0052] According to the CMEImBF prepared in Example 1 4 (0.2mol), n-butylamine 14.6g, (0.2mol), DIC (0.2mol) were added to a 100mL flask, heated to reflux for 1-2h, stopped, washed thoroughly with ether to remove other impurities in the reaction, and then distilled Dry solvent. Get BEImBF 4 , the yield was 89%, and it was a yellow liquid at room temperature.
[0053]
Embodiment 3
[0054] Example 3. Preparation of 1-ethyl-3-(C-(morpholin-4-yl)-formylmethyl)imidazole tetrafluoroborate (MEImBF 4 )
[0055] According to the CMEImBF prepared in Example 1 4 (0.2mol), Morpholine 26.1g, (0.3mol), DIC (0.2mol), CaCl 2 (0.2mol) was added into a 100mL flask, heated to reflux for 10-12 hours, stopped, centrifuged to remove insoluble inorganic salts, and other impurities in the reaction were removed by distillation. Get MEImBF 4 , the yield was 83%, and it was a yellow liquid at room temperature.
[0056]
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com