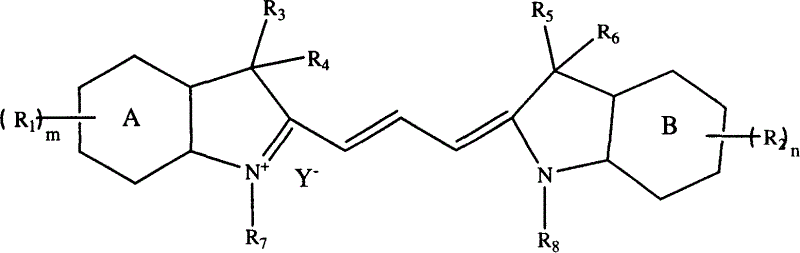
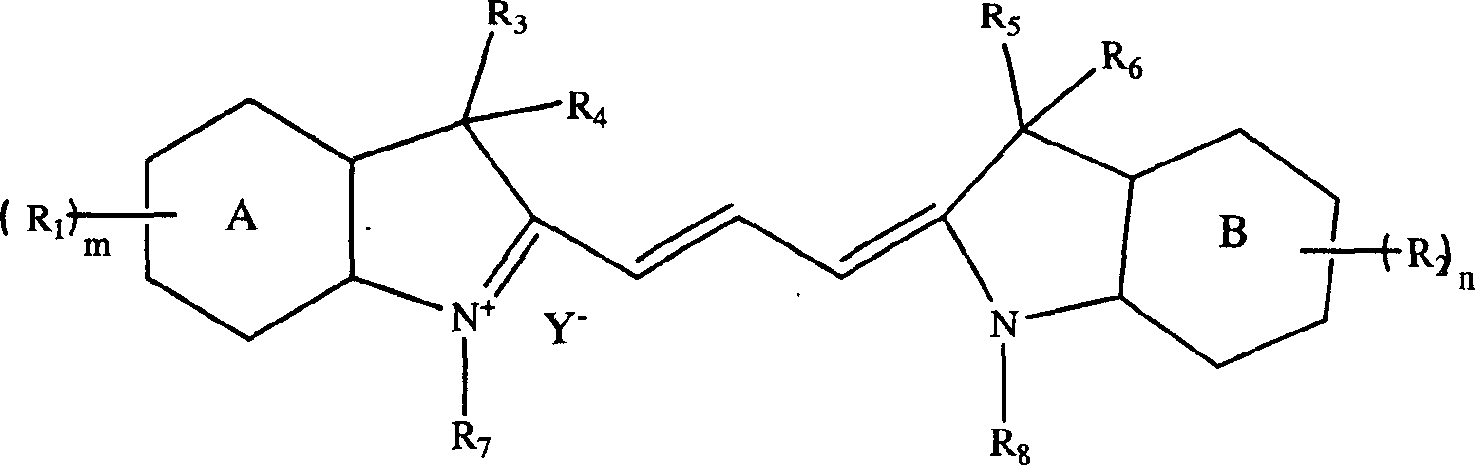
Optical recording dye of trimethyl-methane, and its prepn. method
An optical recording and dye technology, applied in the directions of methine-based/polymethine-based dyes, optical record carriers, record carrier materials, etc., can solve problems such as low purity, and achieve the effect of easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
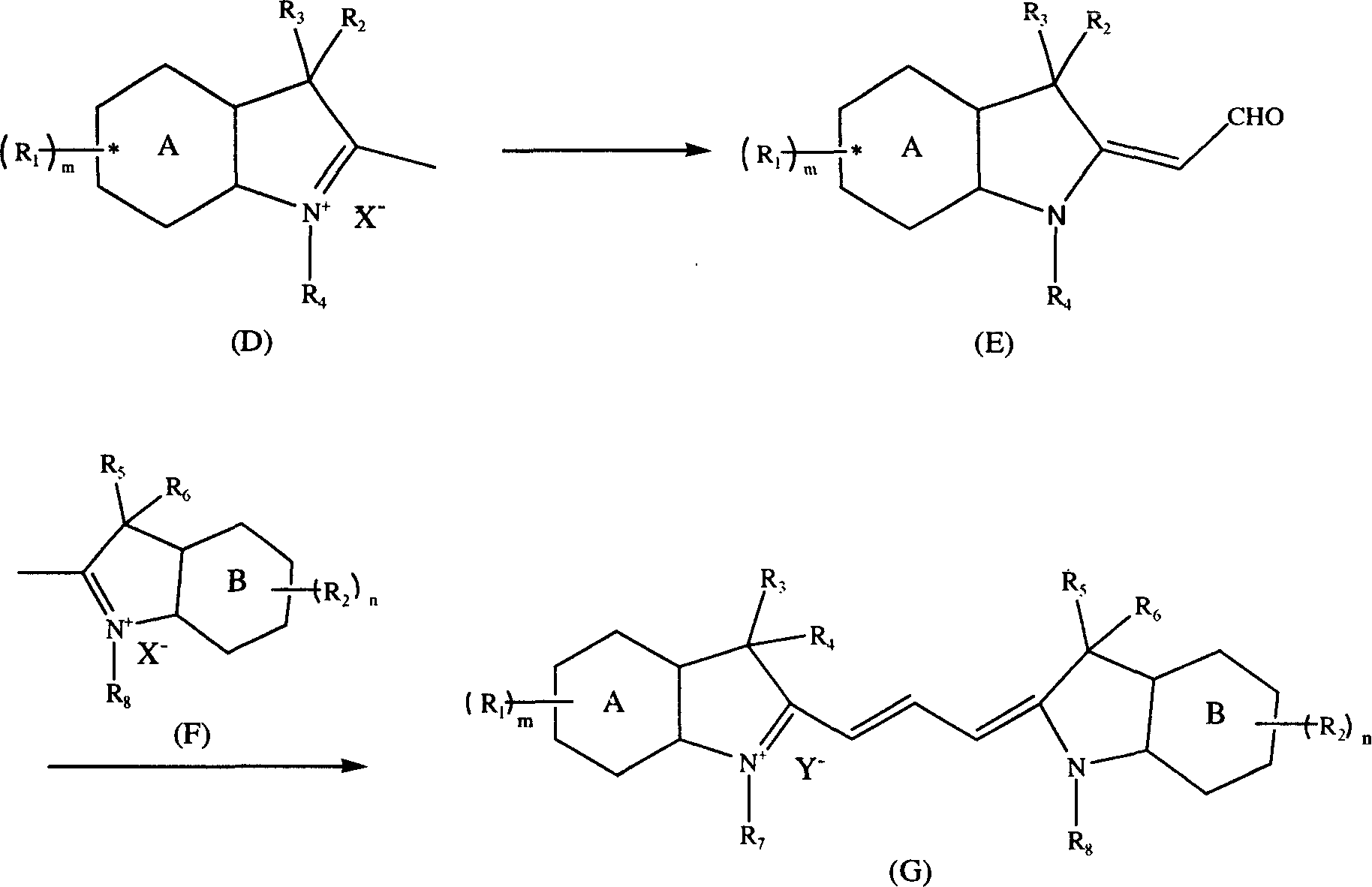
[0039] The preparation of above-mentioned compound generally comprises the following steps:
[0040] (1) Put 1 mol of compound D and a corresponding amount of DMF into the reaction flask, stir and lower the temperature to maintain the temperature of the system at -10°C-50°C, add 1mol-3mol of phosphorus oxychloride dropwise, the reaction exothermic, maintain The temperature is 0°C-50°C. After the dropwise addition, stir for 1hr-5hr, pour into ice water, filter the precipitate, and wash with water until neutral. The solid can be washed with methanol, ethanol, isopropanol, tetrahydrofuran, toluene or Chloroform recrystallization gave intermediate E with a purity of more than 99%, and the yield was 70%-95%.
[0041] (2) Add 1 mol of intermediate E, 1 mol of raw material F, 1 mol of piperidine and 1 mol of acetic anhydride into the reaction flask, raise the temperature to 20°C-100°C, and react for 5 minutes to 5 hours. Pour the reaction product into water, precipitate out, filter,...
Embodiment 1
[0043] Embodiment 1, the preparation of dyestuff No.3
[0044](a) After mixing 152g of 1,2,3,3-tetramethyl-benzo[e]indolium bromide and 500g of N,N-dimethylformamide, add it to a 1000ml reaction bottle, and use ice salt Cool the bath to -5°C, add 77g of phosphorus oxychloride dropwise under stirring, control the temperature below 50°C, stir for 2 hours, pour into 800ml of ice water, stir thoroughly, filter, wash with water until neutral, and dry in vacuum at 50°C to obtain 86.9 g of 1,3,3-trimethyl-2-formylmethylene-benzo[e]indole, yield 91%, purity 99.2%.
[0045] (b) 125.5 g of 1,3,3-trimethyl-2-formylmethylene-benzo[e]indole and 197 g of 1,2,3-trimethyl-3-(2-methyl Base-benzyl)-benzo[e]indolium bromide salt, 43g piperidine and 51g acetic anhydride were added into the reaction flask, heated to 100°C, and reacted for 2 hours. Pour the reaction product into water, precipitate out, filter, wash with water until neutral, and dry. Add the dried solid into 200g of methanol, rai...
Embodiment 2
[0048] Embodiment 2, the preparation of dyestuff No.6
[0049] (a) After mixing 174g 1-isoamyl-2,3,3-trimethyl-benzo[e]indolium bromide and 500g N,N-dimethylformamide, add it to a 1000ml reaction flask , use an ice-salt bath to cool down to 0°C, add 115.5g of phosphorus oxychloride dropwise under stirring, control the temperature below 50°C, stir for 2 hours, pour into 800ml of ice water, stir well, filter, wash with water until neutral, vacuum 50 After drying at °C, 131 g of 1-isoamyl-2-formylmethylene-3,3-dimethyl-benzo[e]indole was obtained, with a yield of 89% and a purity of 99.0%.
[0050] (b) 131 g of 1-isopentyl-2-formylmethylene-3,3-dimethyl-benzo[e]indole and 186.5 g of 1,2-dimethyl-3-spiro- Add cyclohexane-benzo[g]indolium iodide, 43g piperidine, and 51g acetic anhydride into the reaction flask, raise the temperature to 95°C, and react for 3 hours. Pour the reaction product into water, precipitate out, filter, wash with water until neutral, and dry. Add the dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com