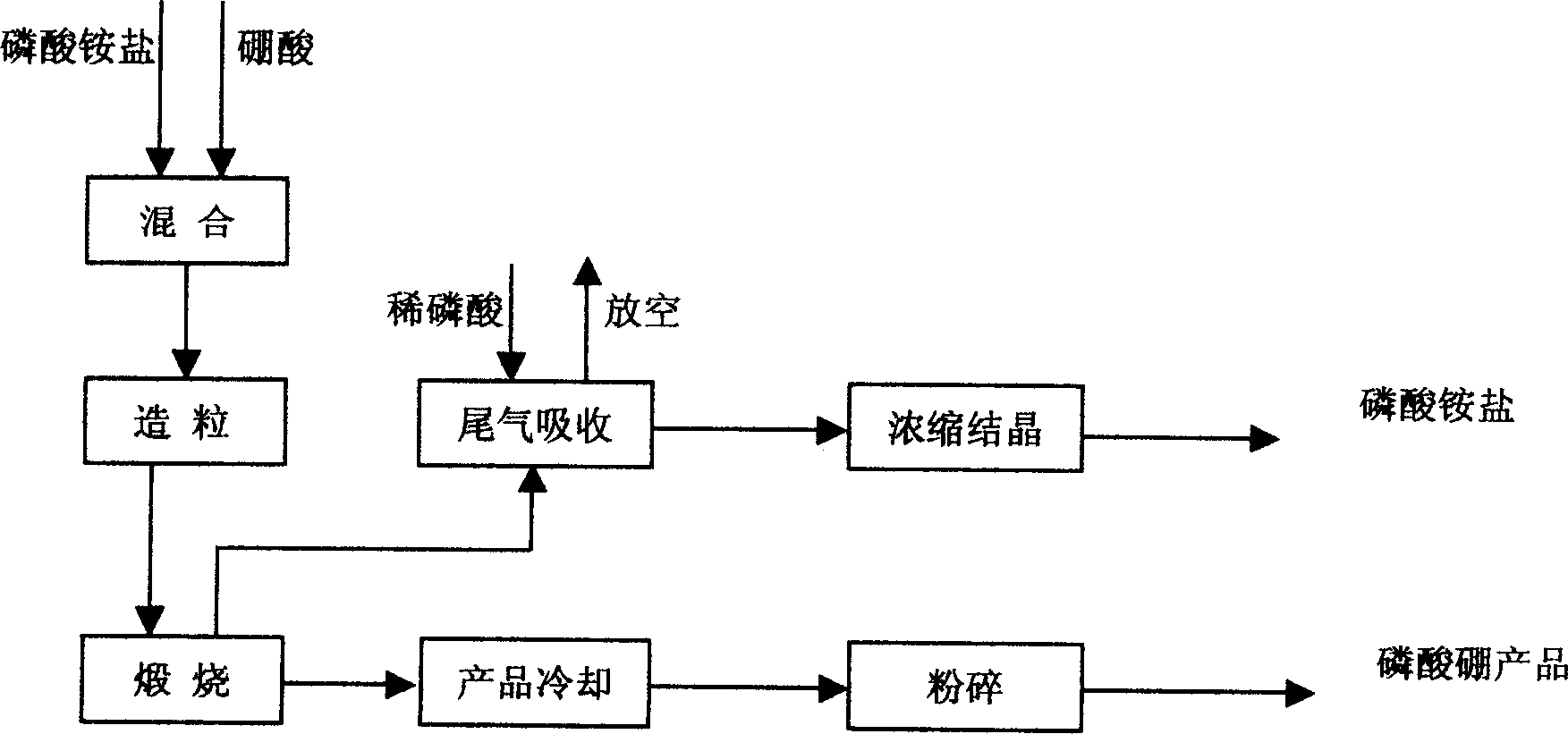
Boron phosphate preparation method
A technology of boron phosphate and phosphoric acid, which is applied in chemical instruments and methods, boron compounds, inorganic chemistry, etc., can solve the problems of complex production control, long process flow, and long reaction time, and achieve simple process flow, short reaction time, and high production efficiency. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] With boric acid as the boron source, ammonium dihydrogen phosphate and phosphoric acid as the phosphorus source, the ingredients are composed of B 2 o 3 :P 2 o 5 =1.1:1 (molar ratio). Add 164Kg of industrial boric acid and 262Kg of industrial ammonium dihydrogen phosphate into the mixer, spray the material with 13.5Kg of industrial phosphoric acid for mixing; send the mixed material into the granulator for extrusion granulation; then send it into the rotary kiln, Calcined at 500°C for 3 hours, then cooled and pulverized the material to obtain the boron phosphate product. Product obtained: B 2 o 3 =32.83%, P 2 o 5 = 63.83%.
Embodiment 2
[0022] With boric acid as the boron source, ammonium dihydrogen phosphate and phosphoric acid as the phosphorus source, the ingredients are composed of B 2 o 3 :P 2 o 5 =1.05:1 (molar ratio). Add 156Kg of industrial boric acid and 262Kg of industrial ammonium dihydrogen phosphate into the mixer, spray the material with 13.5Kg of industrial phosphoric acid for mixing; send the mixed material into the granulator for extrusion granulation; then send it into the rotary kiln, Calcined at 1000°C for 1 hour, then cooled and pulverized the material to obtain the boron phosphate product. Product obtained: B 2 o 3 =32.54%, P 2 o 5 = 65.32%.
Embodiment 3
[0024] With boric acid as the boron source, ammonium dihydrogen phosphate and phosphoric acid as the phosphorus source, the ingredients are composed of B 2 o 3 :P 2 o 5 = 1:1 (molar ratio). Add 149Kg of industrial boric acid and 262Kg of industrial ammonium dihydrogen phosphate into the mixer, spray the material with 13.5Kg of industrial phosphoric acid for mixing; send the mixed material into the granulator for extrusion granulation; then send it into the rotary kiln, Calcined at 800°C for 2 hours, then cooled and pulverized the material to obtain the boron phosphate product. Product obtained: B 2 o 3 =32.13%, P 2 o 5 = 64.59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com