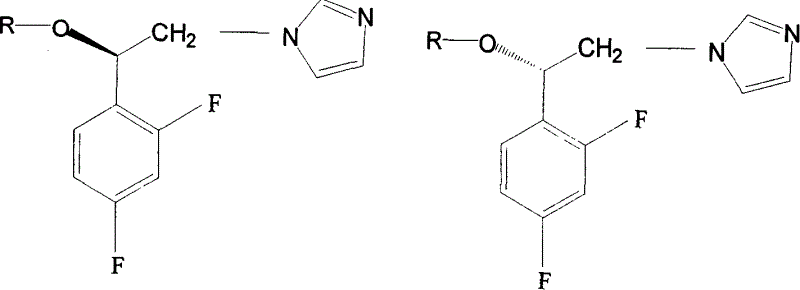
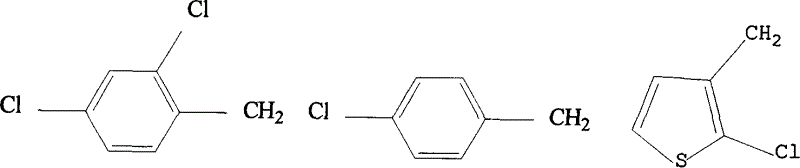
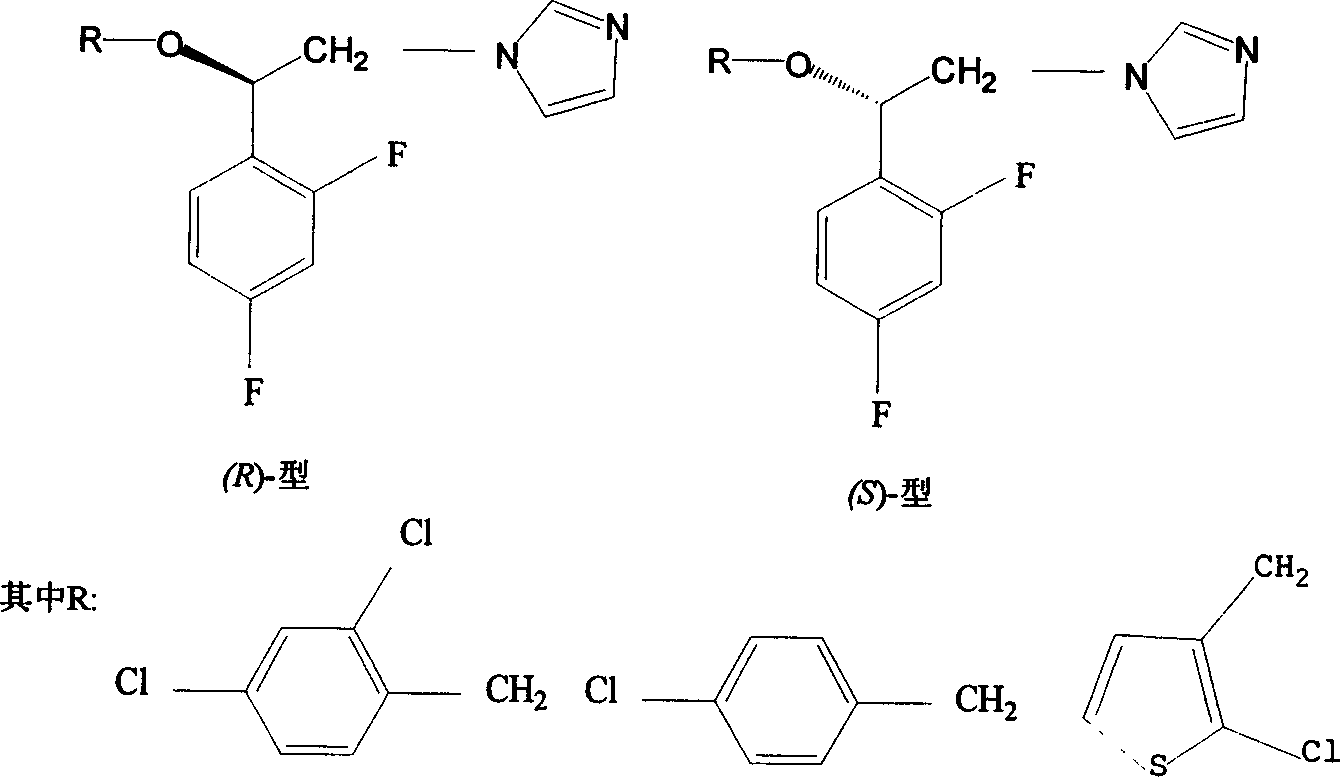
Preparation method of imidazole aromatic alcohol analog derivative with optical activity
An aromatic alcohol, optically active technology, applied in the directions of organic active ingredients, organic chemistry, pharmaceutical formulations, etc., can solve problems such as no optical activity, and achieve the effects of high yield, low cost and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) 2-chloro-2', the preparation of 4'-difluoroacetophenone (2):
[0024] Add 8.667g (0.065mol) of anhydrous aluminum trichloride and 6.45mL (0.065mol) of m-difluorobenzene into a 250mL three-necked flask. At 30°C to 35°C, 3.98 mL (0.050 mol) of chloroacetyl chloride was added dropwise. After the dropwise addition was completed, the temperature was raised to 50°C to 55°C for 4 hours. After the reaction was completed, the mixture was added into 0.6 mol / L 100 mL ice-water hydrochloric acid solution in a thin stream for acid hydrolysis. Filter and wash the solid with water. Recrystallize with n-hexane to obtain light yellow crystals, 8.648g after drying, yield 90.8%. Determination of mp by capillary melting point method: 47℃~48℃.
[0025] (2) Preparation of 2-chloro-1-(2,4-difluorophenyl)-ethanol (3):
[0026] Add 19.05g (0.1mol) of 2-chloro-2',4'-difluoroacetophenone (3) and 90mL of methanol into a 250mL three-necked flask. In an ice-water bath with stirring, NaBH w...
Embodiment 2
[0036] (1) 2-chloro-2', the preparation of 4'-difluoroacetophenone (2):
[0037] Add 8.010g (0.060mol) of anhydrous aluminum trichloride and 6.45mL (0.065mol) of m-difluorobenzene into a 250mL three-necked flask, and in the At 30°C to 35°C, 3.98 mL (0.050 mol) of chloroacetyl chloride was added dropwise. After the dropwise addition was completed, the temperature was raised to 50°C to 55°C for 4 hours. After the reaction was completed, the mixture was added into 0.6 mol / L 100 mL ice-water hydrochloric acid solution in a thin stream for acid hydrolysis. Filter and wash the solid with water. Recrystallize with n-hexane to obtain light yellow crystals, 8.648g after drying, yield 90.8%. Determination of mp by capillary melting point method: 47℃~48℃.
[0038] (2) Preparation of 2-chloro-1-(2,4-difluorophenyl)-ethanol (3):
[0039] With 19.05g (0.1mol) 2-chloro-2', 4'-difluoroacetophenone (3), 22.20g (0.2mol) CaCl 2 , 90mL of methanol was added into a 250mL three-necked flask. ...
Embodiment 3
[0051] (1) 2-chloro-2', the preparation of 4'-difluoroacetophenone (2):
[0052]Add 8.000g (0.060mol) of anhydrous aluminum trichloride and 6.45mL (0.065mol) of m-difluorobenzene into a 250mL three-necked flask. At 30°C to 35°C, 3.98 mL (0.050 mol) of chloroacetyl chloride was added dropwise. After the dropwise addition was completed, the temperature was raised to 30°C-40°C for 4 hours. After the reaction was completed, the mixture was added into 0.6 mol / L 100 mL ice-water hydrochloric acid solution in a thin stream for acid hydrolysis. Filter and wash the solid with water. Recrystallize with n-hexane to obtain light yellow crystals, 8.954 g after drying, yield 94%. Determination of mp by capillary melting point method: 47℃~48℃.
[0053] (2) Preparation of 2-chloro-1-(2,4-difluorophenyl)-ethanol (3):
[0054] With 19.05g (0.1mol) 2-chloro-2', 4'-difluoroacetophenone (2), 19.00g (0.2mol) MgCl 2 , 90mL of methanol was added into a 250mL three-necked flask. Stir at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com