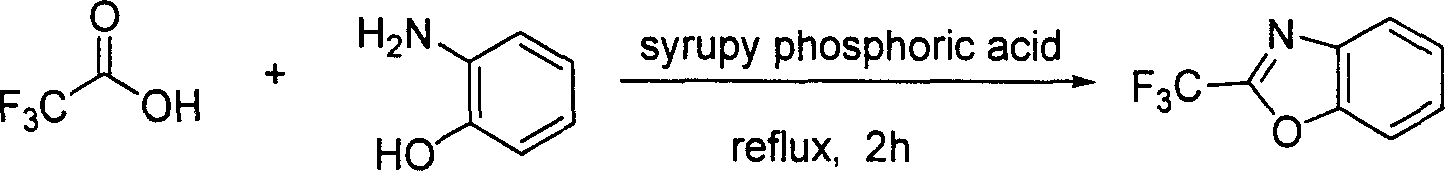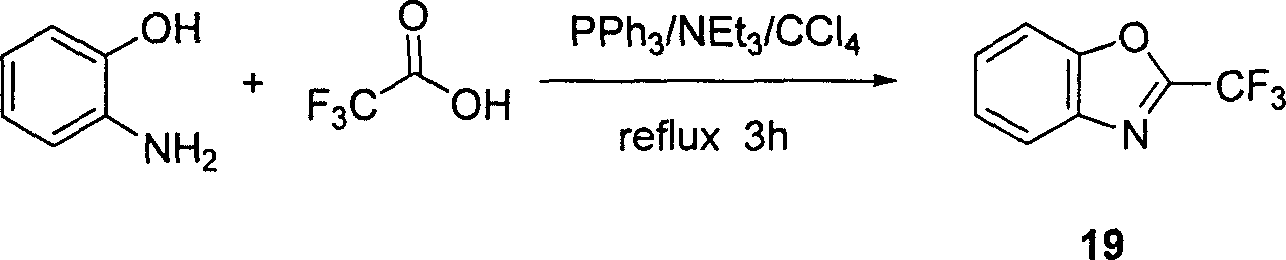Method for preparing 2-trifluoro methyl benzoxazole
A technology of trifluoromethylbenzoxazole and triphenylphosphine, which is applied in the field of preparation of 2-trifluoromethylbenzoxazole, can solve the problems of 2-trifluoromethylbenzoxazole, This type of compound has less research and other problems, and achieves the effect of high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Example one: under nitrogen protection, add triphenylphosphine (34.5 grams, 132 mmoles), triethylamine (18.2 milliliters, 132 millimoles), carbon tetrachloride (21.1 milliliters, 220 milliliters) in the two-necked flask of 250 milliliters millimoles), and a reflux condenser was installed. Cool to 0°C with an ice-water bath while stirring, add trifluoroacetic acid (3.4 ml, 44 mmol) with a syringe through the reversing plug, stir at 0°C for ten minutes, add o-aminophenol (5.8 g, 53 mmol) and carbon tetrachloride solution (21.1 mL, 220 mmol). Slowly heat to 60°C (oil bath temperature), the triphenylphosphine is completely dissolved, and the mixed solution will be relatively clear at this time. Maintain this temperature for a period of time (about 15 minutes), and it can be observed that once the reaction is initiated, the reaction will become very intense, a large amount of heat will be released, violent reflux, and a large amount of white solid will be produced, which is...
example 2
[0016] Example two: under nitrogen protection, add triphenylphosphine (261.37 grams, 1 mole), triethylamine (137.9 milliliters, 1 mole), carbon tetrachloride (159.8 milliliters, 1.67 moles) in a 1-liter two-necked flask , install the reflux condenser. Cool to 0°C with an ice-water bath under stirring, add trifluoroacetic acid (25.76 ml, 0.333 mol) with a syringe through the reversing plug, stir at 0°C for fifteen minutes, add o-aminophenol (44.0 g, 0.402 mol) and Carbon tetrachloride solution (159.8 mL, 1.67 mol). Slowly heat to 60°C (oil bath temperature), the triphenylphosphine is completely dissolved, and the mixed solution will be relatively clear at this time. Maintain this temperature for a period of time (about 20 minutes), and it can be observed that once the reaction is initiated, the reaction will become very intense, a large amount of heat will be emitted, violent reflux, and a large amount of white solid will be produced, which is a salt of triethylamine salt and...
example 3
[0017] Example three: under nitrogen protection, add triphenylphosphine (2.614 kilograms, 10 moles), triethylamine (1.379 liters, 10 moles), carbon tetrachloride (1.598 liters, 16.7 moles) in 10 liters of two-necked flasks , install the reflux condenser. Cool to 0°C with an ice-water bath under stirring, add trifluoroacetic acid (257.6 ml, 3.333 mol) with a syringe through the reversing plug, stir at 0°C for 20 minutes, add o-aminophenol (0.44 kg, 4.015 mol) and Carbon tetrachloride solution (1.598 L, 16.7 moles). Slowly heat to 60°C (oil bath temperature), the triphenylphosphine is completely dissolved, and the mixed solution will be relatively clear at this time. Maintain this temperature for a period of time (about 25 minutes), and it can be observed that once the reaction is initiated, the reaction will become very intense, a large amount of heat will be released, violent reflux, and a large amount of white solid will be produced, which is a salt of triethylamine salt an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com