Anti-chlorine ion corrosion Ni-Cr nano composite coating and preparing method and use
A technology of ion corrosion and nano-composite, applied in the direction of coating, electrolytic coating, etc., to achieve the effect of uniform composition, low porosity and good resistance to Cl ion corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
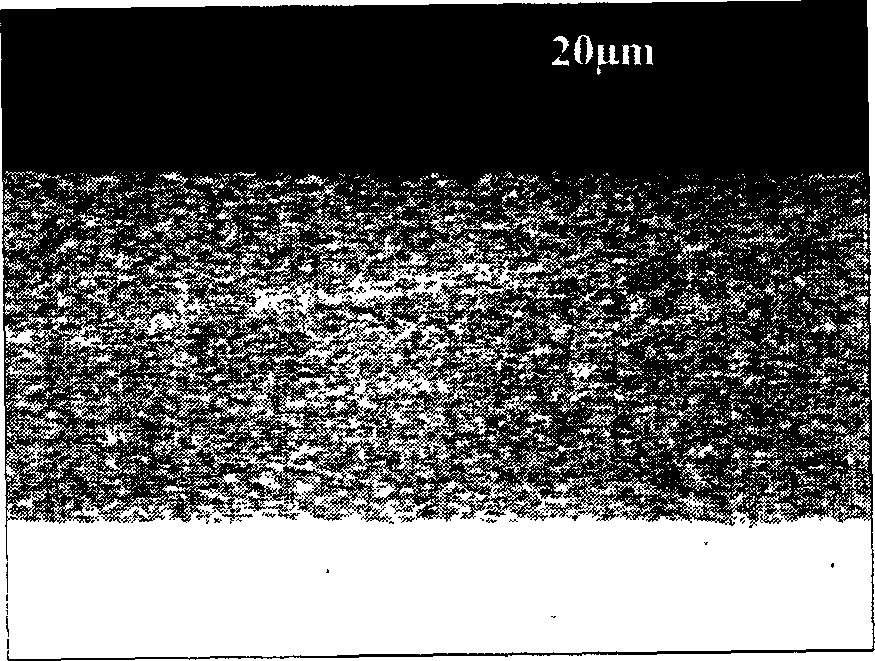
[0040] The preparation method of the Ni-Cr nanocomposite coating is prepared by conventional co-electrodeposition technology, and its process is as follows:
[0041]Substrate metal Ni-surface grinding to 800# water sandpaper-surface ultrasonic cleaning-co-electrodeposition in Ni plating bath containing nano-Cr powder-obtain Ni-Cr nanocomposite coating. The key to the preparation of the nano-coating layer of the present invention is to keep the nano-particles suspended in the bath liquid during electroplating, while having good dispersion performance. The preparation steps of the present invention are specifically as follows:
[0042] Take an electrolytic Ni plate with a purity of 99.96% as the base material, process it into a small sample with a size of 15×10×2mm, and grind it to 800 # , ultrasonic cleaning in acetone;
[0043] Get the nano-Cr powder (prepared by Beijing Zhongkangda Nano Technology Co., Ltd.) with a particle size in the range of 10nm-80nm and an average part...
Embodiment 2
[0048] This example compares the corrosion resistance of Ni-Cr nanocomposite coatings (ENNC: Electrodeposited nanoparticle-dispersed nanocomposite coating) with different Cr contents.
[0049] Using the M273 potentiostat and PAR M352 electrochemical test system produced by EG&G Company, the single Ni coating (ENNC Ni-0Cr), ENNC Ni-4.5Cr and ENNC Ni-10.9Cr (mass percentage, the same below) were electro-conducted, respectively. Chemical corrosion performance test. The experiment adopts a standard three-electrode system: the sample is the working electrode (WE), the platinum sheet is the auxiliary electrode (AE), and the saturated calomel electrode (SCE) is the reference electrode (RE). All potentials are based on it. The experimental data is automatically recorded under the monitoring of the microcomputer, and the software equipped with the M352 system is used for curve fitting and kinetic parameter processing. The experimental temperature is room temperature, and the corrosion...
Embodiment 3
[0051] This example compares the corrosion resistance of ENNC Ni-Cr and Ni-Cr coating of composite micron-sized particles (EMCC: Electrodeposited microparticle-dispersed composite coating).
[0052] In order to further study the corrosion resistance of ENNC, the potentiodynamic polarization curves and constant potential I-t curves of ENNC Ni-10.9Cr and EMCC Ni-12.4Cr were tested respectively by using the same equipment, the same parameters and the standard three-electrode system. Figure 7 It is the test result of potentiodynamic polarization curve. It can be seen that in the case of similar Cr content, the pitting breakdown potential and passivation range of ENNC Ni-10.9Cr is greater than that of EMCC Ni-12.4Cr (Table 2), so the pitting corrosion resistance of ENNC Ni-10.9C is better than that of EMCC Ni-12.4Cr. Figure 8 It is the I-t curve of the two coatings in the passivation interval (E=-0.1V). It can be seen that the anode current density of the ENNNCi-10.9Cr passivat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com