Manufacturing method of erbium doped high silicon oxygen infrared luminous glass
A technology of infrared light emission and manufacturing method, applied in glass manufacturing equipment, glass molding, manufacturing tools, etc., can solve the problems of unfavorable development of miniaturization of devices, small gain per unit length of optical fiber, low erbium ion concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
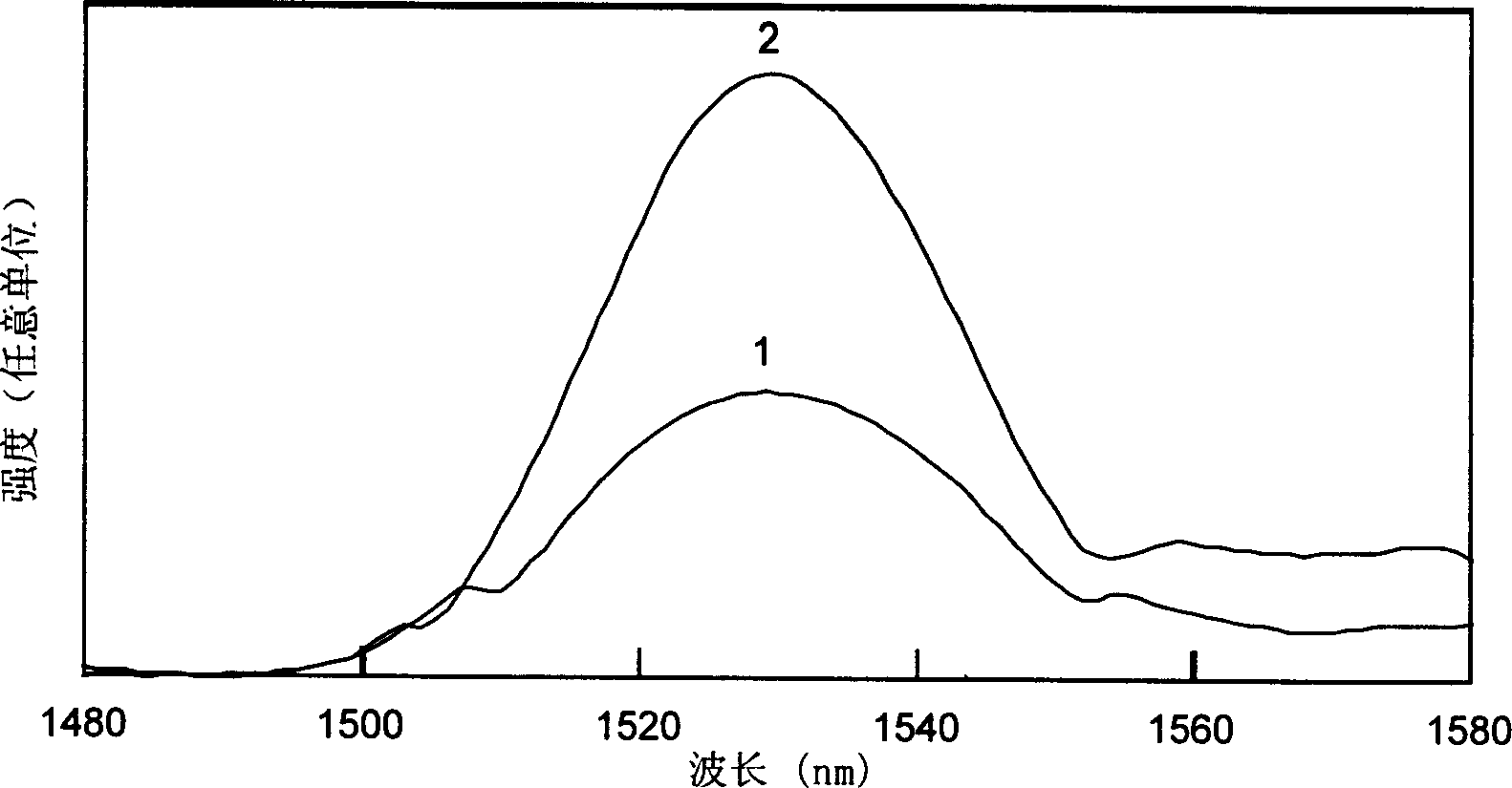
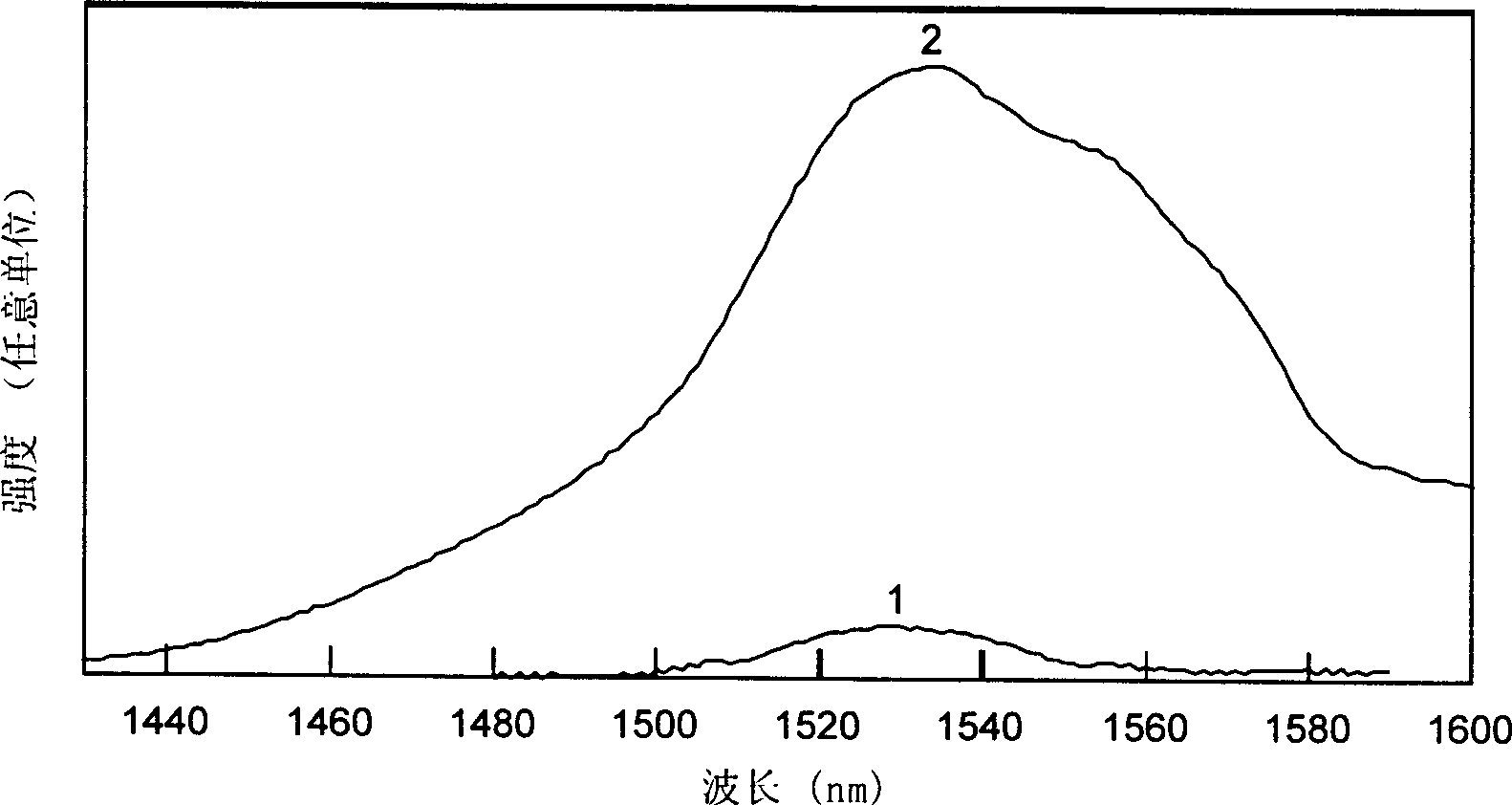
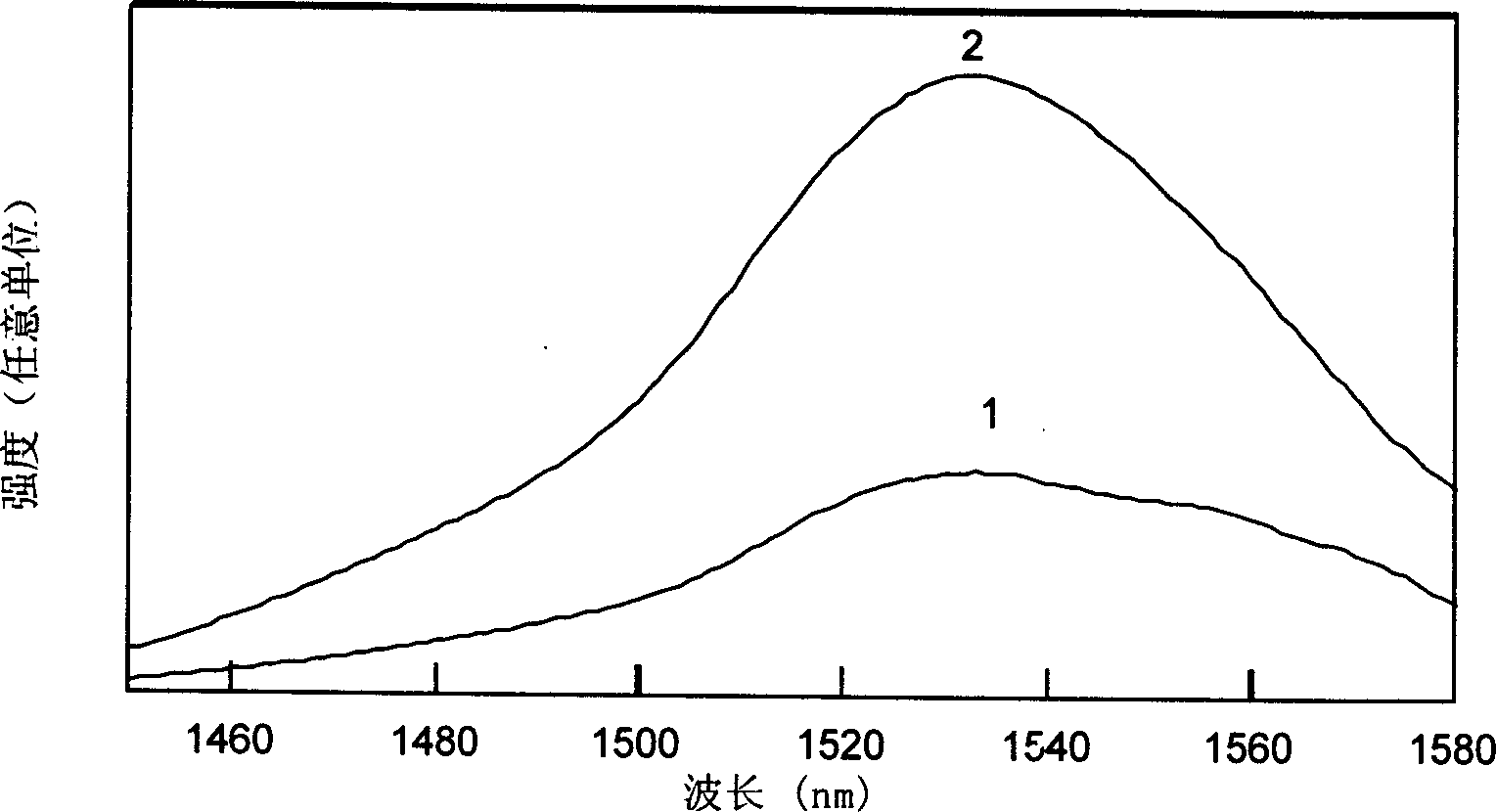
[0023] Will be decomposed equivalent to 1.0g of Er 2 o 3 2.32g of analytically pure Er(NO 3 ) 3 ·5H 2 O was put into 25 ml of deionized water solution, and after it was completely dissolved, it was formulated as Er 3+ A solution with an ion concentration of 0.21 mol / L, and a size of 5×5×3mm, SiO 2 The porous glass with a content of 97wt% is put into the solution and soaked for more than 10 minutes; after that, the high-silica porous glass doped with erbium ions is put into a high-temperature furnace, and undergoes solid-state sintering at 1150°C in air or oxygen to eliminate Micropores become dense and transparent doped Er 2 o 3 A high silica glass with a concentration of approximately 1.0%. During the sintering process, after rising from room temperature to 400°C at a speed of less than 5°C per minute, it rises to around 950°C at a speed of 10°C per minute, and then rises from this temperature at a speed of below 5°C per minute. After reaching 1150°C and keeping the t...
Embodiment 2
[0025] After decomposing, it is equivalent to 0.1g of Er 2 o 3 0.23g of analytically pure Er(NO 3 ) 3 ·5H 2 O is put into 25 ml of ethanol solution, after it is completely dissolved, it is dubbed Er 3+ A solution with an ion concentration of 0.02 mol / L, and a size of 5×5×3mm, SiO 2 The porous glass with a content of 98wt% is put into the solution and soaked for more than 10 minutes; after that, the high-silica microporous glass is put into a high-temperature furnace, and undergoes solid-phase sintering at a temperature of 1200°C in air or oxygen to eliminate Micropores become dense and transparent doped Er 2 o 3 High silica glass with a concentration of about 0.1%. During the sintering process, after rising from room temperature to 400°C at a speed of less than 5°C per minute, it rises to around 950°C at a speed of 10°C per minute, and then rises from this temperature at a speed of below 5°C per minute. After reaching 1200°C and keeping the temperature at this temperat...
Embodiment 3
[0027] Will be decomposed equivalent to 1.0g of Er 2 o 3 2.32g of analytically pure Er(NO 3 ) 3 ·5H 2 O and 5.0 g of analytically pure Al(NO 3 ) 3 9H 2 O is put into 25 ml of 1 N hydrochloric acid solution, after it is completely dissolved, it is dubbed Er 3+ The ion concentration is 0.21 mol / L, Al 3+ A solution with an ion concentration of 0.53 mol / L, and a size of 5×5×3mm, SiO 2The porous glass with a content of 97wt% is put into the solution and soaked for more than 10 minutes; after that, the high-silica microporous glass doped with these ions is put into a high-temperature furnace, and passes through a solid phase at a temperature of 1120°C in air or oxygen. Sintering eliminates micropores and becomes dense and transparent high-silica glass. During the sintering process, the temperature is raised from room temperature to 400°C at a speed of less than 5°C per minute, and then raised to 950°C at a speed of 10°C per minute. Then, raise from 950°C to 1120°C at a spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com